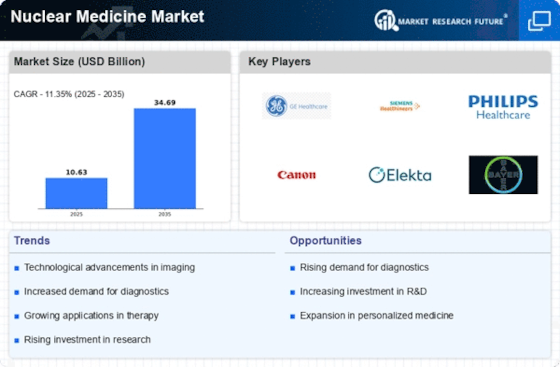
Top Industry Leaders in the Nuclear Medicine Market

Latest Nuclear Medicine Companies Update:
Curium Acquired IASON, an Austrian radiopharmaceutical company, expanding their diagnostic product portfolio in Europe. Received FDA approval for the production of Pluvicto (177Lu-PSMA-617) in Indianapolis, providing increased capacity for this important prostate cancer theranostic agent. Collaborating with research institutions on developing novel radiopharmaceuticals for various diseases.
Lantheus Holdings, Launched their PYLARIFY (F-18 PSMA-1007) injectable, the first commercially available PSMA PET imaging agent for prostate cancer in the US. Investing in research on next-generation radiopharmaceuticals for oncology and other therapeutic areas.
Siemens Healthineers Introduced their Biograph Vision Quadra PET/CT scanner, offering improved image quality and faster scan times. Collaborating with various companies and research institutions on developing theranostic solutions and advancing clinical applications of nuclear medicine.
GE Healthcare Launched their Discovery NM 750i SPECT/CT system, featuring advanced detector technology and improved image resolution. Focusing on developing AI-powered image analysis tools for nuclear medicine procedures.
Blue Earth Diagnostics Signed a data-sharing agreement with Siemens Healthineers and the University Hospital of the Technical University of Munich (TUM) for 18F-rhPSMA-7.3, an investigational PET imaging agent for prostate cancer, to support AI-based algorithms development. Conducting clinical trials for their radiopharmaceutical candidates targeting cancers and other diseases.
List of Nuclear Medicine companies in the market
- Cardinal Health (US)
- GE Healthcare (US)
- Bayer AG (Germany)
- Bracco Imaging (Italy)
- Medtronic Plc (Ireland)
- Lantheus Medical Imaging (US)
- Nordion, Inc. (Canada)
- Advanced Accelerators Applications (France)
- Mallinckrodt Pharmaceuticals (UK)
- Jubilant Lifesciences (India)