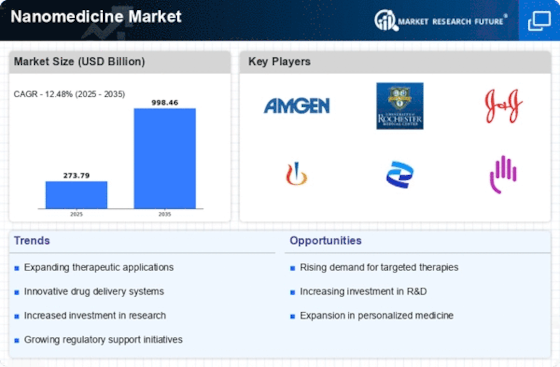
Top Industry Leaders in the Nanomedicine Market
 Latest Nanomedicine Companies Update
Latest Nanomedicine Companies Update
Feb 2023: On Friday, Sun Pharmaceutical Industries announced that the US health agency had permitted its company to distribute a generic drug to treat multiple myeloma. According to a statement from Sun Pharma, the US Food and Drug Administration has given the business final approval for the generic lenalidomide capsules in various dosages. Sun Pharma and Celgene Corporation reached a deal in June 2021 to end the patent litigation about Sun Pharma's generic lenalidomide capsules. As part of the settlement agreement, Celgene licensed Sun Pharma the patents needed to produce and market specific, restricted amounts of generic lenalidomide capsules in the United States starting in March 2022.
Nov 2023: Pfizer and Serina Therapeutics have a license agreement for Pfizer's small-molecule drug delivery technology. Serina's POZ technology, which comprises lipid nanoparticle delivery mechanisms for RNA-based treatments, will now be available to Pfizer. Pfizer will receive a non-exclusive license from Serina to use its POZ platform medication delivery technology. The synthetic, low-viscosity polymer poly(2-oxazoline) is the foundation for Serina's POZ platform based in the United States. The platform releases drugs through enzymatic means. Using a cleavable linker, a medication is first bonded to side groups of the polymer, according to the company website. The plasma enzyme butyrylcholinesterase then releases the active medication.
List of Nanomedicine Key companies in the market
- Pfizer Inc. (US)
- Johnson & Johnson Services, Inc., (US)
- General Electric Company (US)
- Invitae Corporation (US)
- Leadient BioSciences Inc (Italy)
- Merck & Co., Inc. (US)
- DiaSorin S.p.A. (Italy)
- Nanosphere Health Sciences, Inc. (US)
- Celgene Corporation (US)
- Teva Pharmaceutical Industries Ltd. (Israel)