Market Analysis
In-depth Analysis of Mendelian Disorders Testing Market Industry Landscape
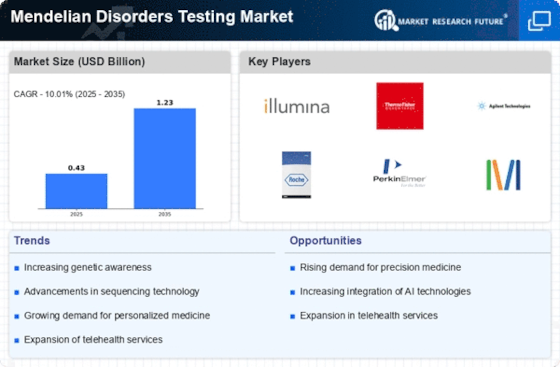
The Mendelian Disorders Testing market is experiencing noteworthy trends that are reshaping the landscape of genetic diagnostics. One prominent trend is the increasing adoption of genetic testing for Mendelian disorders, driven by advancements in DNA sequencing technologies. The ability to rapidly and cost-effectively sequence an individual's entire genome or specific gene regions has revolutionized the diagnosis of Mendelian disorders, providing healthcare professionals with unprecedented insights into the genetic basis of these conditions.
Technological advancements, particularly in next-generation sequencing (NGS), are playing a pivotal role in shaping market trends. NGS allows for high-throughput sequencing, enabling the simultaneous analysis of multiple genes associated with Mendelian disorders. This has significantly reduced the time and cost involved in genetic testing, making it more accessible to a broader population. The trend towards more efficient and affordable testing methods is facilitating widespread adoption, leading to earlier and more accurate diagnoses of Mendelian disorders.
Another key trend is the expanding scope of Mendelian disorders testing beyond research settings to routine clinical practice. As the clinical utility and reliability of genetic testing have improved, healthcare providers are increasingly integrating genetic testing into their diagnostic workflows. This trend is transforming the standard of care for patients with suspected Mendelian disorders, allowing for personalized treatment plans based on the specific genetic mutations identified through testing.
Furthermore, the market is witnessing a growing emphasis on comprehensive genetic testing panels that cover a wide range of Mendelian disorders. These panels, which include hundreds or even thousands of genes associated with various genetic conditions, provide a more holistic approach to genetic testing. Healthcare professionals can leverage these panels to simultaneously assess a patient's risk for multiple Mendelian disorders, streamlining the diagnostic process and optimizing the use of genetic information in clinical decision-making.
The rise of direct-to-consumer genetic testing services is another significant trend in the Mendelian Disorders Testing market. Increasing consumer awareness and interest in their genetic makeup have led to the popularity of at-home DNA testing kits. While these services offer insights into ancestry and traits, they also provide information about potential genetic predispositions to certain Mendelian disorders. This trend is contributing to the democratization of genetic information, empowering individuals to take proactive measures for their health and facilitating the identification of potential genetic risks.
Collaboration and partnerships between genetic testing companies, healthcare providers, and pharmaceutical firms are also shaping market trends. These collaborations aim to enhance the integration of genetic testing results into patient care, facilitate research on Mendelian disorders, and accelerate the development of targeted therapies. The synergies created by these partnerships contribute to a more comprehensive and patient-centric approach to managing Mendelian disorders.
Despite the positive trends, challenges such as ethical considerations, data privacy concerns, and the interpretation of genetic variants remain important considerations in the Mendelian Disorders Testing market. Striking a balance between providing valuable genetic information and ensuring the responsible use of that information is crucial for maintaining public trust and advancing the field.

















Leave a Comment