Market Trends
Key Emerging Trends in the Medical Device Packaging Market
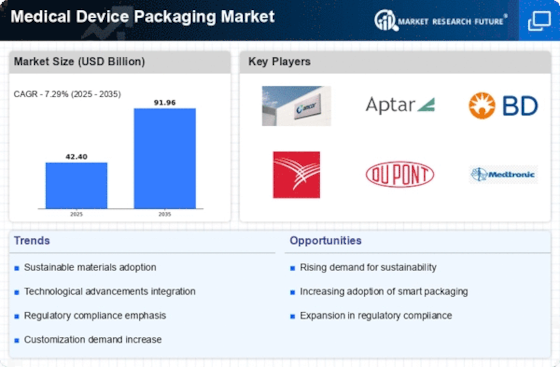
The global medical device packaging market has been experiencing significant trends driven by factors such as advancements in medical technology, changing regulatory landscapes, and evolving consumer preferences. Medical device packaging plays a crucial role in ensuring the safety, sterility, and integrity of medical devices throughout their lifecycle, from manufacturing to end-use in healthcare facilities and homes.
Businesses have to abide by the law of the land and cannot operate outside it. The rigid policies of medical authorities to provide standardized medical devices may hinder the growth of the market globally. This is an obstacle for medical device packaging material manufacturers. The package of medical devices is generally made up of plastic. Minimizing the use of plastic is also a challenge for companies.
One notable trend in the global medical device packaging market is the increasing demand for packaging solutions that prioritize product protection and patient safety. With the growing complexity and diversity of medical devices, there is a heightened focus on packaging designs that provide adequate protection against contamination, damage, and tampering. This trend is driven by regulatory requirements aimed at ensuring the efficacy and safety of medical devices, as well as consumer expectations for high-quality, reliable packaging that safeguards their health and well-being.
Moreover, technological advancements have been driving innovation in the medical device packaging market, leading to the development of advanced materials and packaging technologies. Manufacturers are investing in research and development to introduce materials with enhanced barrier properties, compatibility with sterilization methods, and sustainability features. Additionally, advancements in packaging machinery and automation are enabling the production of packaging solutions that meet stringent quality standards, improve efficiency, and reduce costs.
Additionally, the global medical device packaging market is influenced by changing regulatory landscapes and industry standards governing packaging and labeling practices. Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe establish guidelines and requirements for medical device packaging to ensure compliance with safety, sterility, and labeling standards. Compliance with these regulations drives the adoption of packaging solutions that meet specific criteria for materials, design, and labeling, ensuring product safety and regulatory compliance.
Furthermore, market trends in the global medical device packaging market are shaped by shifting consumer preferences and industry dynamics. With an increasing emphasis on sustainability and environmental responsibility, there is a growing demand for eco-friendly packaging solutions made from recyclable materials or featuring biodegradable properties. Manufacturers are responding to this demand by offering sustainable packaging options that minimize environmental impact while maintaining performance and compliance with regulatory requirements. This trend reflects the growing awareness and importance of sustainability considerations in medical device packaging practices.
















Leave a Comment