Growing Patient Safety Awareness
The rising awareness of patient safety is a significant driver in the Medical Device Complaint Management Market. As patients become more informed about their rights and the potential risks associated with medical devices, they are more likely to report complaints. This trend is prompting manufacturers to enhance their complaint management systems to address concerns proactively. According to recent studies, nearly 60% of patients express a desire for better communication regarding device-related issues. Consequently, companies are investing in training programs and resources to ensure that their staff is equipped to handle complaints effectively, thereby fostering a culture of safety and responsiveness.
Emphasis on Regulatory Compliance
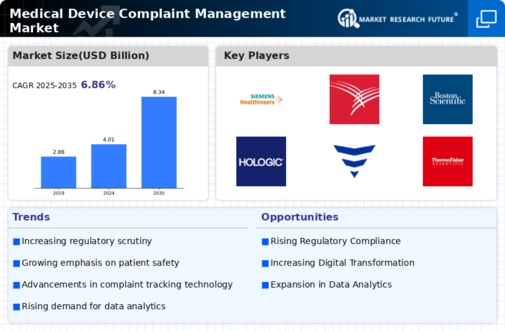
Regulatory compliance remains a critical driver in the Medical Device Complaint Management Market. Stringent regulations imposed by authorities such as the FDA and EMA necessitate that manufacturers maintain rigorous complaint management systems. Non-compliance can result in severe penalties, including product recalls and legal actions, which can significantly impact a company's reputation and financial standing. The market is witnessing an increase in investments towards compliance management solutions, with a projected growth rate of 12% annually. This trend indicates that companies are prioritizing the establishment of robust complaint management frameworks to ensure adherence to regulatory standards, thereby enhancing their operational integrity and consumer trust.
Increased Focus on Data Analytics
The increased focus on data analytics is shaping the Medical Device Complaint Management Market by enabling organizations to derive actionable insights from complaint data. By leveraging analytics, companies can identify patterns and root causes of complaints, which can inform product improvements and risk management strategies. The market for data analytics in healthcare is expected to grow at a CAGR of 23% over the next five years, reflecting the critical role of data-driven decision-making in enhancing complaint management processes. This emphasis on analytics not only aids in compliance with regulatory requirements but also enhances overall product quality and patient satisfaction.
Integration of Advanced Technologies
The integration of advanced technologies such as artificial intelligence and machine learning is transforming the Medical Device Complaint Management Market. These technologies facilitate the automation of complaint handling processes, enabling quicker response times and improved accuracy in data analysis. As a result, companies can identify trends and potential issues more effectively, leading to enhanced patient safety and satisfaction. The market for AI in healthcare is projected to reach USD 45 billion by 2026, indicating a robust growth trajectory that underscores the importance of technological advancements in complaint management. Furthermore, the adoption of cloud-based solutions allows for real-time data access and collaboration among stakeholders, which is crucial for timely resolution of complaints.
Digital Transformation in Complaint Handling
Digital transformation is reshaping the Medical Device Complaint Management Market by streamlining processes and enhancing communication channels. The shift towards digital platforms allows for more efficient tracking and management of complaints, reducing the time taken to resolve issues. Companies are increasingly adopting integrated software solutions that provide comprehensive dashboards for monitoring complaint trends and outcomes. This transition is supported by a growing demand for transparency and accountability in healthcare, with 70% of consumers indicating a preference for companies that demonstrate effective complaint resolution practices. As digital tools become more prevalent, organizations are likely to experience improved customer engagement and loyalty.