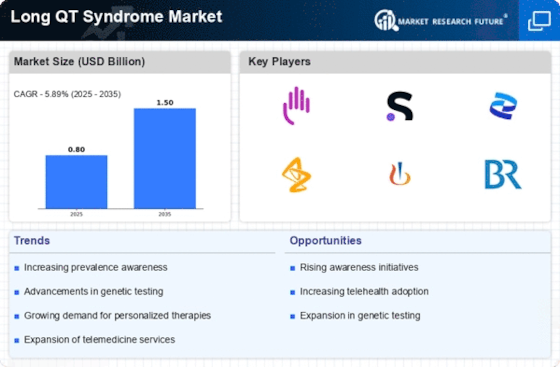
Top Industry Leaders in the Long QT Syndrome Market

Latest Long QT Syndrome Companies Updates
January 2024: Actelion Pharmaceuticals (a Janssen company) announced the initiation of a Phase 3 clinical trial assessing the efficacy and safety of their potassium sodium adenosine triphosphatase (Na/K-ATPase) inhibitor verapamil in preventing syncope (fainting) episodes in LQTS patients.
November 2023: The US Food and Drug Administration (FDA) granted Breakthrough Therapy Designation to Cohero BioScience's CBP-501 for reducing mortality and morbidity in infants with LQTS. This designation expedites the development and review process for the drug.
August 2023: Biotronik received CE Mark approval for their Lumax HF7 ICD (implantable cardioverter defibrillator) designed specifically for pediatric patients with LQTS. This approval provides a smaller, more comfortable device option for younger LQTS patients.
December 2023: Invitae, a genetic testing company, partnered with the LQTS Foundation to expand access to genetic testing for LQTS. This partnership aims to improve early diagnosis and management of the condition.
October 2023: The Mayo Clinic and Pfizer entered into a strategic collaboration to develop and commercialize personalized medicine solutions for LQTS. This collaboration leverages Mayo Clinic's expertise in clinical care and research with Pfizer's drug development capabilities.
List of Long QT Syndrome Key companies in the market:
- Invitae Corporation (U.S.)
- GeneDx. (U.S.)
- Asper Biogene (Estonia)
- Boston Scientific Corporation (U.S.)
- Laboratory Corporation of America Holdings (U.S.)
- Pfizer Inc. (U.S.)
- Zydus Pharmaceuticals, Inc. (U.S.)
- Aralez Pharmaceuticals Inc. (Canada)
- AstraZeneca (U.K)
- Torrent Pharmaceuticals Limited (India)
- Lupin Pharmaceuticals, Inc. (U.S.)
- Cipla Inc. (India)