Growing Awareness and Advocacy
Growing awareness and advocacy for Dravet syndrome are pivotal drivers of the dravet syndrome market. Patient advocacy groups in Japan are actively working to educate the public and healthcare professionals about the challenges faced by individuals with this condition. Increased awareness leads to earlier diagnosis and intervention, which is crucial for managing symptoms effectively. Moreover, these advocacy efforts often result in greater funding for research and development, as well as improved healthcare policies. The heightened visibility of Dravet syndrome may also encourage pharmaceutical companies to invest in the market, recognizing the potential for new therapies. Consequently, the dravet syndrome market is likely to expand as more stakeholders become engaged in addressing the needs of affected individuals.
Advancements in Genetic Research
Advancements in genetic research are significantly influencing the dravet syndrome market. With the identification of specific genetic mutations associated with Dravet syndrome, there is a growing emphasis on targeted therapies. In Japan, research institutions are increasingly focusing on understanding the genetic underpinnings of this condition, which could lead to the development of personalized medicine approaches. The potential for gene therapy and other innovative treatments is becoming more apparent, as clinical trials are underway to evaluate their efficacy. This focus on genetic research not only enhances treatment options but also raises awareness about the condition, potentially increasing patient enrollment in clinical studies. As a result, the dravet syndrome market is likely to experience growth driven by these scientific advancements.
Government Initiatives and Support
Government initiatives and support play a crucial role in shaping the dravet syndrome market in Japan. The Japanese government has implemented various policies aimed at improving healthcare access for patients with rare diseases, including Dravet syndrome. These initiatives often include financial assistance for treatment and incentives for pharmaceutical companies to develop new therapies. For instance, the orphan drug designation provides benefits such as market exclusivity and tax incentives, which can stimulate research and development efforts. As a result, the dravet syndrome market is likely to benefit from increased investment in innovative treatments and improved patient access to existing therapies. This supportive regulatory environment may encourage more companies to enter the market, further enhancing treatment options for patients.
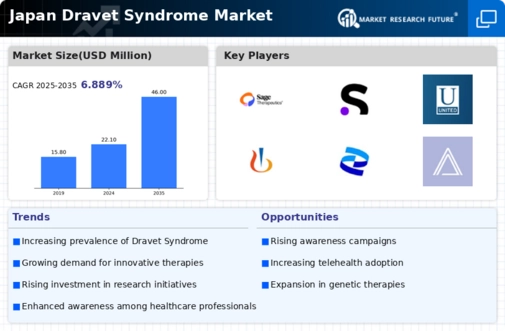
Rising Incidence of Dravet Syndrome
The increasing incidence of Dravet syndrome in Japan is a notable driver for the dravet syndrome market. Recent studies indicate that the prevalence of this rare genetic epilepsy disorder is approximately 1 in 15,700 live births. This rising incidence necessitates enhanced healthcare services and treatment options, thereby expanding the market. As awareness grows among healthcare professionals and families, the demand for effective therapies is likely to increase. Furthermore, the Japanese government has been proactive in recognizing rare diseases, which may lead to improved funding and support for research and development in the dravet syndrome market. This trend suggests a potential for growth in both the pharmaceutical and healthcare sectors, as more patients seek specialized care and innovative treatments.
Emergence of Innovative Treatment Options
The emergence of innovative treatment options is transforming the landscape of the dravet syndrome market. Recent developments in antiepileptic drugs and novel therapies, such as cannabidiol (CBD) products, have shown promise in managing seizures associated with Dravet syndrome. In Japan, the approval of new medications has provided patients with additional choices, enhancing their quality of life. The ongoing research into combination therapies and personalized medicine approaches further indicates a shift towards more effective treatment paradigms. As these innovative options become available, the dravet syndrome market is likely to witness increased demand from patients and healthcare providers seeking better management strategies. This trend suggests a dynamic and evolving market landscape, driven by the need for effective solutions.