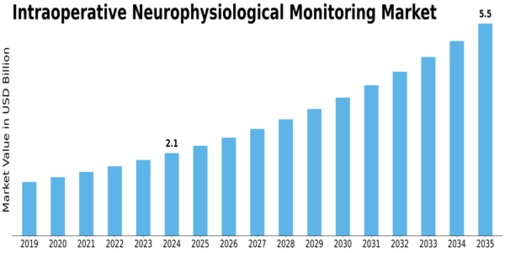
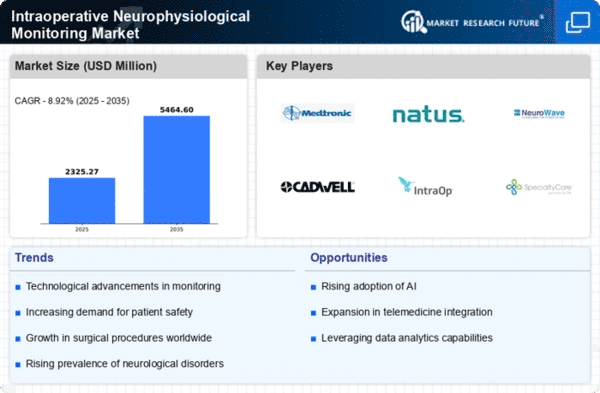
Intraoperative Neurophysiological Monitoring Size
Intraoperative Neurophysiological Monitoring Market Growth Projections and Opportunities
The electroencephalography (EEG) segment emerged as the largest in the intraoperative neurophysiological monitoring market, recording a revenue of $670.1 million in 2015. It is projected to escalate to $1816.1 million by 2027, exhibiting a growth rate of 8.6% from 2016 to 2027. Simultaneously, electromyography (EMG) is identified as the fastest-growing segment, anticipated to experience a remarkable 9.3% compound annual growth rate (CAGR) during the forecast period.
Within the type category, EEG dominated the market, securing the largest market share in 2015, generating $670.1 million in revenue. This segment is on a trajectory to reach $1816.1 million by 2027, reflecting an 8.6% CAGR. Conversely, EMG is emerging as the fastest-growing segment, poised for an impressive 9.3% CAGR over the forecasted period.
Examining the intraoperative neurophysiological monitoring market by procedure, the spinal segment took the lead, accruing $748.4 million in revenue in 2015. Projections indicate an upswing to $2052.6 million by 2027, growing at a rate of 8.7% from 2016 to 2027. In tandem, orthopedic procedures are identified as the fastest-growing segment, expected to expand at a rate of 9.3% from 2016 to 2027.
In terms of procedures, spinal interventions held the largest market share, amassing $748.4 million in revenue in 2015. Forecasts suggest a substantial increase to $2052.6 million by 2027, reflecting an 8.7% CAGR. Simultaneously, orthopedic procedures are identified as the fastest-growing segment, poised for a noteworthy 9.3% CAGR during the forecasted period.
Segmenting the intraoperative neurophysiological monitoring market by method, the invasive segment exhibited dominance with a revenue of $687.5 million in 2015. Projections signal an upward trajectory, reaching $1862.7 million by 2027, growing at a rate of 8.6% from 2016 to 2027. In parallel, the non-invasive segment stands out as the fastest-growing, poised to expand at a rate of 9.3% from 2016 to 2027.
Examining the market by method, the invasive approach garnered the largest market share, amassing $687.5 million in revenue in 2015. Forecasts predict a robust increase to $1862.7 million by 2027, reflecting an 8.6% CAGR. Simultaneously, the non-invasive method emerges as the fastest-growing segment, poised for a noteworthy 9.3% CAGR during the forecasted period.
By source, the in-house segment led the intraoperative neurophysiological monitoring market, generating $670.4 million in revenue in 2015. Projections indicate an upward trajectory, reaching $1828.3 million by 2027, with a growth rate of 8.7% from 2016 to 2027. Meanwhile, the outsourced segment is identified as the fastest-growing, expected to expand at a rate of 9.2% from 2016 to 2027.
Analyzing the market by source, the in-house segment exhibited dominance, securing the largest market share with $670.4 million in revenue in 2015. Forecasts suggest substantial growth to $1828.3 million by 2027, reflecting an 8.7% CAGR. Concurrently, the outsourced segment emerges as the fastest-growing, anticipated to expand at an impressive 9.2% CAGR during the forecasted period.







Leave a Comment