-
EXECUTIVE SUMMARY
-
MARKET INTRODUCTION
-
SCOPE OF THE STUDY 17
-
RESEARCH OBJECTIVE 17
-
MARKET STRUCTURE 17
-
ASSUMPTIONS & LIMITATIONS 18
-
RESEARCH METHODOLOGY
-
DATA MINING 19
-
SECONDARY RESEARCH 20
-
PRIMARY RESEARCH 21
-
BREAKDOWN OF PRIMARY RESPONDENTS 22
-
FORECASTING TECHNIQUES 23
-
RESEARCH METHODOLOGY FOR MARKET SIZE ESTIMATION 24
- BOTTOM-UP APPROACH 25
- TOP-DOWN APPROACH 25
-
DATA TRIANGULATION 26
-
VALIDATION 26
-
MARKET DYNAMICS
-
OVERVIEW 27
-
DRIVERS 29
- LARGE NUMBER OF PATIENTS SUFFERING FROM BRAIN CANCER, ANEURYSM, AND RELATED CONDITIONS 29
- GROWING NUMBER OF INCIDENCES OF TRAUMA AND ACCIDENTS LEADING TO TRAUMATIC BRAIN INJURY (TBI) 29
- CLINICAL TRIALS TO IMPROVE AVAILABLE TREATMENTS 30
- INCREASING HEALTHCARE EXPENDITURE 30
-
RESTRAINTS 31
- INCREASING COUNTERFEIT DRUGS & SIDE EFFECTS OF THE TREATMENT 31
- LACK OF SKILLED WORKFORCE 32
-
OPPORTUNITIES 33
- NEW PRODUCT LAUNCH IN DEVELOPING REGIONS 33
-
MARKET FACTOR ANALYSIS
-
VALUE CHAIN ANALYSIS 34
- R&D 35
- MANUFACTURING 35
- DISTRIBUTION AND SALES 35
- POST-SALES MONITORING 35
-
PORTER’S FIVE FORCES MODEL 36
- BARGAINING POWER OF SUPPLIERS 37
- BARGAINING POWER OF BUYERS 37
- THREAT OF NEW ENTRANTS 37
- THREAT OF SUBSTITUTES 37
- INTENSITY OF RIVALRY 37
-
COVID-19 IMPACT ANALYSIS 38
- IMPACT ON GROWTH RATE 38
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE
-
OVERVIEW 39
-
INTRACEREBRAM HEMORRHAGE 40
-
SUBARACHNOID HEMORRHAGE 40
-
EPIDURAL HEMATOMA 41
-
SUBDURAL HEMATOMA 41
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS & TREATMENT
-
OVERVIEW 42
-
DIAGNOSIS 43
-
TREATMENT 44
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY REGION
-
OVERVIEW 46
-
AMERICAS 47
- NORTH AMERICA 50
- LATIN AMERICA 58
-
EUROPE 60
- WESTERN EUROPE 63
- EASTERN EUROPE 78
-
ASIA-PACIFIC 80
- CHINA 83
- JAPAN 85
- INDIA 87
- AUSTRALIA 89
- SOUTH KOREA 91
- REST OF ASIA-PACIFIC 93
-
MIDDLE EAST & AFRICA 96
- MIDDLE EAST 99
- AFRICA 101
-
COMPETITIVE LANDSCAPE
-
OVERVIEW 103
-
COMPETITIVE ANALYSIS 103
-
COMPETITIVE BENCHMARKING 104
-
LEADING PLAYERS IN TERMS OF NUMBER OF DEVELOPMENTS 105
-
MAJOR GROWTH STRATEGY IN THE GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET 105
-
KEY DEVELOPMENTS & GROWTH STRATEGIES 106
- PRODUCT APPROVALS 106
- ACQUISITIONS 106
- AGREEMENTS/COLLABORATIONS 106
-
COMPANY PROFILES
-
CANON INC. 107
- COMPANY OVERVIEW 107
- FINANCIAL OVERVIEW 108
- PRODUCTS/SERVICES OFFERED 109
- KEY DEVELOPMENTS 109
- SWOT ANALYSIS 110
- KEY STRATEGIES 110
-
FUJIFILM CORPORATION 111
- COMPANY OVERVIEW 111
- FINANCIAL OVERVIEW 112
- PRODUCTS/SERVICES OFFERED 112
- KEY DEVELOPMENTS 112
- SWOT ANALYSIS 113
- KEY STRATEGIES 113
-
GE HEALTHCARE 114
- COMPANY OVERVIEW 114
- FINANCIAL OVERVIEW 115
- PRODUCTS/SERVICES OFFERED 116
- KEY DEVELOPMENTS 116
- SWOT ANALYSIS 117
- KEY STRATEGIES 117
-
HITACHI, LTD 118
- COMPANY OVERVIEW 118
- FINANCIAL OVERVIEW 118
- PRODUCTS/SERVICES OFFERED 119
- KEY DEVELOPMENTS 119
- SWOT ANALYSIS 120
- KEY STRATEGIES 120
-
INFRASCAN, INC. 121
- COMPANY OVERVIEW 121
- FINANCIAL OVERVIEW 121
- PRODUCTS/SERVICES OFFERED 121
- KEY DEVELOPMENTS 121
- SWOT ANALYSIS 122
- KEY STRATEGIES 122
-
JOHNSON & JOHNSON 123
- COMPANY OVERVIEW 123
- FINANCIAL OVERVIEW 123
- PRODUCTS/SERVICES OFFERED 124
- KEY DEVELOPMENTS 124
- SWOT ANALYSIS 125
- KEY STRATEGIES 125
-
KONINKLIJKE PHILIPS NV 126
- COMPANY OVERVIEW 126
- FINANCIAL OVERVIEW 127
- PRODUCTS/SERVICES OFFERED 128
- KEY DEVELOPMENTS 128
- SWOT ANALYSIS 129
- KEY STRATEGIES 129
-
MEDTRONIC PLC 130
- COMPANY OVERVIEW 130
- FINANCIAL OVERVIEW 130
- PRODUCTS/SERVICES OFFERED 131
- KEY DEVELOPMENTS 131
- SWOT ANALYSIS 132
- KEY STRATEGIES 132
-
SIEMENS 133
- COMPANY OVERVIEW 133
- FINANCIAL OVERVIEW 134
- PRODUCTS/SERVICES OFFERED 135
- KEY DEVELOPMENTS 135
- SWOT ANALYSIS 136
- KEY STRATEGIES 136
-
STRYKER 137
- COMPANY OVERVIEW 137
- FINANCIAL OVERVIEW 137
- PRODUCTS/SERVICES OFFERED 138
- KEY DEVELOPMENTS 138
- SWOT ANALYSIS 139
- KEY STRATEGIES 139
-
APPENDIX
-
REFERENCES 140
-
RELATED REPORTS 140
-
LIST OF TABLES
-
LIST OF ASSUMPTIONS & LIMITATIONS 18
-
PRIMARY INTERVIEWS AND INFORMATION GATHERING PROCESS 21
-
AVAILABILITY OF NEUROSURGEONS IN DEVELOPING ASIAN COUNTRIES, 2023 32
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 40
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET FOR INTRACEREBRAM HEMORRHAGE, BY REGION, 2023-2032 (USD MILLION) 40
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET FOR SUBARACHNOID HEMORRHAGE, BY REGION, 2023-2032 (USD MILLION) 40
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET FOR EPIDURAL HEMATOMA, BY REGION, 2023-2032 (USD MILLION) 41
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET FOR SUBDURAL HEMATOMA, BY REGION, 2023-2032 (USD MILLION) 41
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 42
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY REGION, 2023-2032 (USD MILLION) 43
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 43
-
GLOBAL INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY REGION, 2023-2032 (USD MILLION) 44
-
GLOBAL INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 45
-
GLOBAL INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 45
-
GLOBAL INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 45
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY REGION, 2023-2032 (USD MILLION) 46
-
AMERICAS: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY REGION, 2023-2032 (USD MILLION) 48
-
AMERICAS: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 48
-
AMERICAS: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 49
-
AMERICAS: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 49
-
AMERICAS: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 49
-
AMERICAS: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 49
-
AMERICAS: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 50
-
NORTH AMERICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY COUNTRY, 2023-2032 (USD MILLION) 51
-
NORTH AMERICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 51
-
NORTH AMERICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 51
-
NORTH AMERICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 52
-
NORTH AMERICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 52
-
NORTH AMERICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 52
-
NORTH AMERICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 53
-
US: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 53
-
US: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 54
-
US: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 54
-
US: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 54
-
US: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 55
-
US: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 55
-
CANADA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 56
-
CANADA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 56
-
CANADA: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 56
-
CANADA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 57
-
CANADA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 57
-
CANADA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 57
-
LATIN AMERICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 58
-
LATIN AMERICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 58
-
LATIN AMERICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 58
-
LATIN AMERICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 59
-
LATIN AMERICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 59
-
LATIN AMERICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 59
-
EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY REGION, 2023-2032 (USD MILLION) 60
-
EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 61
-
EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 61
-
EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 61
-
EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 62
-
EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 62
-
EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 62
-
WESTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY COUNTRY, 2023-2032 (USD MILLION) 63
-
WESTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 64
-
WESTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 64
-
WESTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 64
-
WESTERN EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 65
-
WESTERN EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 65
-
WESTERN EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 65
-
GERMANY: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 66
-
GERMANY: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 66
-
GERMANY: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 66
-
GERMANY: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 67
-
GERMANY: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 67
-
GERMANY: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 67
-
FRANCE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 68
-
FRANCE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 68
-
FRANCE: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 68
-
FRANCE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 69
-
FRANCE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 69
-
FRANCE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 69
-
UK: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 70
-
UK: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 70
-
UK: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 70
-
UK: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 71
-
UK: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 71
-
UK: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 71
-
ITALY: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 72
-
ITALY: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 72
-
ITALY: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 72
-
ITALY: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 73
-
ITALY: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 73
-
ITALY: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 73
-
SPAIN: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 74
-
SPAIN: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 74
-
SPAIN: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 74
-
SPAIN: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 75
-
SPAIN: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 75
-
SPAIN: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 75
-
REST OF WESTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 76
-
REST OF WESTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 76
-
REST OF WESTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 76
-
REST OF WESTERN EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 77
-
REST OF WESTERN EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 77
-
REST OF WESTERN EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 77
-
EASTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 78
-
EASTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 78
-
EASTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 78
-
EASTERN EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 79
-
EASTERN EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 79
-
EASTERN EUROPE: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 79
-
ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY COUNTRY, 2023-2032 (USD MILLION) 81
-
ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 81
-
ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 81
-
ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 82
-
ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 82
-
ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 82
-
ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 83
-
CHINA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 83
-
CHINA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 83
-
CHINA: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 84
-
CHINA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 84
-
CHINA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 84
-
CHINA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 85
-
JAPAN: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 85
-
JAPAN: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 85
-
JAPAN: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 86
-
JAPAN: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 86
-
JAPAN: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 86
-
JAPAN: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 87
-
INDIA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 87
-
INDIA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 87
-
INDIA: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 88
-
INDIA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 88
-
INDIA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 88
-
INDIA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 89
-
AUSTRALIA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 89
-
AUSTRALIA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 89
-
AUSTRALIA: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 90
-
AUSTRALIA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 90
-
AUSTRALIA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 90
-
AUSTRALIA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 91
-
SOUTH KOREA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 91
-
SOUTH KOREA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 91
-
SOUTH KOREA: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 92
-
SOUTH KOREA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 92
-
SOUTH KOREA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 92
-
SOUTH KOREA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 93
-
REST OF ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 93
-
REST OF ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 93
-
REST OF ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 94
-
REST OF ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 94
-
REST OF ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 94
-
REST OF ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 95
-
MIDDLE EAST & AFRICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY REGION, 2023-2032 (USD MILLION) 96
-
MIDDLE EAST & AFRICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 97
-
MIDDLE EAST & AFRICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 97
-
MIDDLE EAST & AFRICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 97
-
MIDDLE EAST & AFRICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 98
-
MIDDLE EAST & AFRICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 98
-
MIDDLE EAST & AFRICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 98
-
MIDDLE EAST: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 99
-
MIDDLE EAST: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 99
-
MIDDLE EAST: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 99
-
MIDDLE EAST: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 100
-
MIDDLE EAST: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 100
-
MIDDLE EAST: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 100
-
AFRICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 101
-
AFRICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS AND TREATMENT, 2023-2032 (USD MILLION) 101
-
AFRICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS MARKET, BY TYPE, 2023-2032 (USD MILLION) 101
-
AFRICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023-2032 (USD MILLION) 102
-
AFRICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR MEDICATION, BY TYPE, 2023-2032 (USD MILLION) 102
-
AFRICA: INTRACRANIAL HEMORRHAGE TREATMENT MARKET FOR SURGERY, BY TYPE, 2023-2032 (USD MILLION) 102
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, COMPANY RANKING ANALYSIS, 2023 103
-
TOP PLAYERS IN TERMS OF NUMBER OF DEVELOPMENTS IN THE INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET 105
-
PRODUCT APPROVALS/LAUNCHES 106
-
ACQUISITIONS 106
-
COLLABORATIONS 106
-
CANON INC.: PRODUCTS/SERVICES OFFERED 109
-
CANON INC.: KEY DEVELOPMENTS 109
-
FUJIFILM CORPORATION: PRODUCTS/SERVICES OFFERED 112
-
GE HEALTHCARE: PRODUCTS/SERVICES OFFERED 116
-
HITACHI, LTD: PRODUCTS/SERVICES OFFERED 119
-
HITACHI, LTD: KEY DEVELOPMENTS 119
-
INFRASCAN, INC.: PRODUCTS/SERVICES OFFERED 121
-
JOHNSON & JOHNSON: PRODUCTS/SERVICES OFFERED 124
-
KONINKLIJKE PHILIPS NV: PRODUCTS/SERVICES OFFERED 128
-
KONINKLIJKE PHILIPS NV: KEY DEVELOPMENTS 128
-
MEDTRONIC PLC: PRODUCTS/SERVICES OFFERED 131
-
SIEMENS: PRODUCTS/SERVICES OFFERED 135
-
SIEMENS: KEY DEVELOPMENTS 135
-
STRYKER: PRODUCTS/SERVICES OFFERED 138
-
STRYKER: KEY DEVELOPMENTS 138
-
LIST OF FIGURES
-
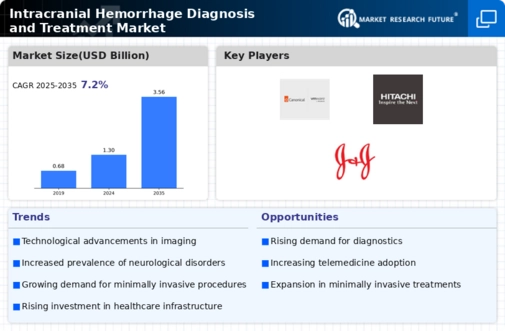
MARKET SYNOPSIS 16
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET 17
-
BOTTOM-UP AND TOP-DOWN APPROACHES 24
-
MARKET DYNAMICS: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET 28
-
DRIVER IMPACT ANALYSIS 31
-
RESTRAINT IMPACT ANALYSIS 33
-
VALUE CHAIN ANALYSIS: GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET 34
-
PORTER’S FIVE FORCES ANALYSIS: GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET 36
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY TYPE, 2023 AND 2032 (USD MILLION) 39
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY DIAGNOSIS & TREATMENT, 2023 AND 2032 (USD MILLION) 42
-
GLOBAL INTRACRANIAL HEMORRHAGE TREATMENT MARKET, BY TYPE, 2023 AND 2032 (USD MILLION) 44
-
GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY REGION, 2023 AND 2032 (USD MILLION) 46
-
AMERICAS: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY REGION, 2023 AND 2032 (USD MILLION) 48
-
NORTH AMERICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY COUNTRY, 2023 AND 2032 (USD MILLION) 50
-
EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY REGION, 2023 AND 2032 (USD MILLION) 60
-
WESTERN EUROPE: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY COUNTRY, 2023 AND 2032 (USD MILLION) 63
-
ASIA-PACIFIC: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY COUNTRY, 2023 AND 2032 (USD MILLION) 80
-
MIDDLE EAST & AFRICA: INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET, BY REGION, 2023 AND 2032 (USD MILLION) 96
-
BENCHMARKING OF MAJOR COMPETITORS 104
-
MAJOR STRATEGY ADOPTED BY KEY PLAYERS IN THE GLOBAL INTRACRANIAL HEMORRHAGE DIAGNOSIS & TREATMENT MARKET 105
-
CANON INC.: FINANCIAL OVERVIEW SNAPSHOT 108
-
CANON INC.: SWOT ANALYSIS 110
-
FUJIFILM CORPORATION: FINANCIAL OVERVIEW SNAPSHOT 112
-
FUJIFILM CORPORATION: SWOT ANALYSIS 113
-
GE HEALTHCARE: FINANCIAL OVERVIEW SNAPSHOT 115
-
GE HEALTHCARE: SWOT ANALYSIS 117
-
HITACHI, LTD: FINANCIAL OVERVIEW SNAPSHOT 118
-
HITACHI, LTD: SWOT ANALYSIS 120
-
INFRASCAN, INC.: SWOT ANALYSIS 122
-
JOHNSON & JOHNSON: FINANCIAL OVERVIEW SNAPSHOT 123
-
JOHNSON & JOHNSON: SWOT ANALYSIS 125
-
KONINKLIJKE PHILIPS NV: FINANCIAL OVERVIEW SNAPSHOT 127
-
-
KONINKLIJKE PHILIPS NV: SWOT ANALYSIS 129
-
MEDTRONIC PLC: FINANCIAL OVERVIEW SNAPSHOT 130
-
MEDTRONIC PLC: SWOT ANALYSIS 132
-
SIEMENS: FINANCIAL OVERVIEW SNAPSHOT 134
-
SIEMENS: SWOT ANALYSIS 136
-
STRYKER: FINANCIAL OVERVIEW SNAPSHOT 137
-
STRYKER: SWOT ANALYSIS 139





Leave a Comment