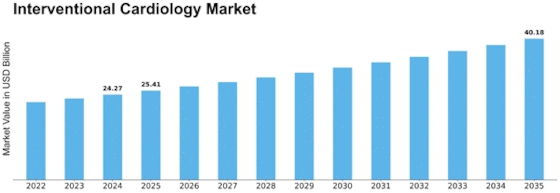
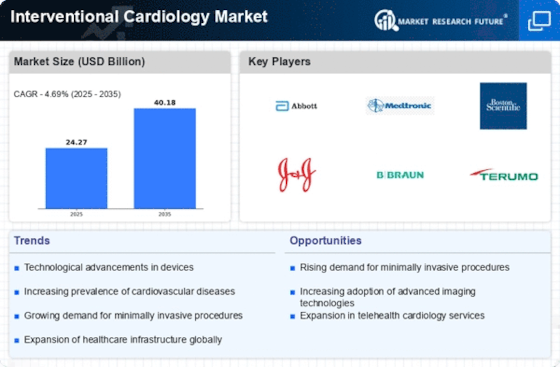
Interventional Cardiology Size
Interventional Cardiology Market Growth Projections and Opportunities
The global cardiovascular disease population affects the invasive cardiology market. Invasive cardiology treatments and equipment are expanding as more individuals suffer heart ailments including coronary artery disease and myocardial infarction.
New technology improve interventional cardiac therapies. New technologies like drug-eluting stents, better imaging, and less invasive therapies make surgeries safer and more successful, attracting physicians and patients.
The aging population affects invasive cardiology. Elderly adults get cardiovascular disease more often. More patients need angioplasty and stents, growing the market. Inactivity, bad nutrition, and smoking raise cardiovascular disease rates. As risk factors stay stable, interventional cardiac therapy rise, reducing market growth. Government initiatives and healthcare policies to prevent and cure cardiovascular diseases impact the interventional cardiology business. Public health, education, and heart-healthy treatment funding impact market variables. Interventional cardiac procedures are becoming increasingly popular because patients prefer painless treatments. More minimally invasive procedures like percutaneous cardiac therapies boost the market since they recover quicker and have fewer complications. Interventional cardiology is rising because more individuals can access medical treatment, particularly in poorer countries. Better hospitals and public awareness allow more patients to get diagnostic and invasive cardiac therapies. Market trends evolve. How invasive cardiac therapies are reimbursed affects the market. Favorable payment policies encourage healthcare staff and patients to receive these therapies. It boosts the market. Travel and global medical services boost the interventional cardiology industry. Patients who go abroad for high-quality, affordable medical care constitute a diverse and increasing market. Invasive cardiology uses AI to enhance diagnosis and operation success. AI algorithms aid in image interpretation, planning, and decision-making. They boost market growth and patient care. Invasive cardiology is trending toward bioresorbable stents. The breakdown of these stents may reduce long-term issues and the necessity for permanent implantation. This might alter customer preferences and inspire new ideas. The interventional cardiology market advances from clinical trials. Investigational research, particularly those on new tools and procedures, shape the market by introducing new solutions and enhancing existing approaches. Medical technologies and healthcare collaborations effect interventional cardiology. Tech, pharma, and healthcare businesses working together may generate new ideas, reach more consumers, and improve competition. Companies must obey government laws and acquire authorization to employ new invasive cardiology techniques to maintain market stability. Following the guidelines helps develop innovative therapies, protects patients, and fosters company confidence.
















Leave a Comment