

- Global Market Outlook
- In-depth analysis of global and regional trends
- Analyze and identify the major players in the market, their market share, key developments, etc.
- To understand the capability of the major players based on products offered, financials, and strategies.
- Identify disrupting products, companies, and trends.
- To identify opportunities in the market.
- Analyze the key challenges in the market.
- Analyze the regional penetration of players, products, and services in the market.
- Comparison of major players’ financial performance.
- Evaluate strategies adopted by major players.
- Recommendations
- Vigorous research methodologies for specific market.
- Knowledge partners across the globe
- Large network of partner consultants.
- Ever-increasing/ Escalating data base with quarterly monitoring of various markets
- Trusted by fortune 500 companies/startups/ universities/organizations
- Large database of 5000+ markets reports.
- Effective and prompt pre- and post-sales support.
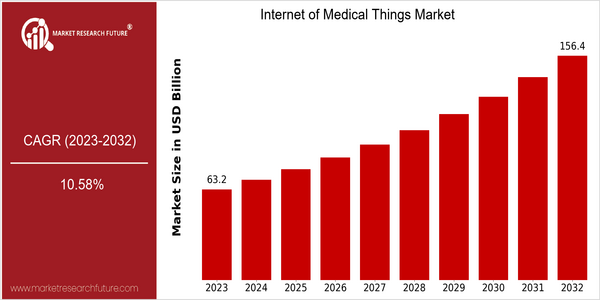
Internet Medical Things Market Size Snapshot
| Year | Value |
|---|---|
| 2023 | USD 63.25 Billion |
| 2032 | USD 156.4 Billion |
| CAGR (2024-2032) | 10.58 % |
Note – Market size depicts the revenue generated over the financial year
The IoT in Medicine market is expected to reach $63 billion in 2023 and reach $ 156 billion by 2032. This represents a CAGR of 10.26% between 2023 and 2032. The main driver of this growth is the integration of advanced digital technologies such as artificial intelligence, machine learning and big data in health systems. These new digital tools enhance patient monitoring, improve operational efficiency and improve health outcomes, thereby increasing the adoption of IoT in Medicine solutions. The main players in the IoT in Medicine industry, including Philips Health, Medtronic and GE Health, are investing in new product development and strategic alliances to take advantage of this growing market. Medtronic, for example, has recently entered into a partnership with several tech companies to enhance its remote patient monitoring capabilities, while Philips has focused on expanding its connected health solutions portfolio. These initiatives not only illustrate the growing competition in the industry but also reflect the growing trend of digital transformation in the health sector, which is expected to increase the growth of the IoT in Medicine market.
Regional Deep Dive
The IoMT market is growing in many regions. It is driven by technological advances, rising health costs and the need for patient-centric care. In North America, the market is characterized by high penetration of connected medical devices, a robust health system and high investment in digital health. Europe is seeing a rise in regulatory frameworks that support the integration of IoMT, while Asia-Pacific is growing rapidly due to increasing health expenditure and the growing population. Middle East and Africa are adopting IoMT slowly, partly driven by government initiatives, while Latin America is starting to adopt IoMT as part of its health system modernization.
North America
- A new set of guidelines for digital health devices from the Food and Drug Administration (FDA) will simplify the approval process for IoMT devices, which will facilitate their development and entry into the market.
- The big companies, such as Philips and Medtronic, are investing heavily in the IoMT, focusing on remote patient monitoring and chronic disease management. This is reshaping the way care is provided.
- The COVID-19 pandemic has caused a significant increase in the use of telemedical services and connected devices, and has led to a lasting change in the way health care is delivered in the region.
Europe
- The European Union’s Medical Device Regulation (MDR) imposes a higher standard of conformity on manufacturers, pushing them to ensure the safety and efficacy of their IoMT devices.
- European Connected Health Alliance is promoting cooperative projects to integrate IoMT into existing health systems, thereby facilitating interoperability and data sharing.
- Germany and the United Kingdom are investing in digital health. For example, the NHS Long-Term Plan emphasizes the use of IoMT to improve patient outcomes.
Asia-Pacific
- The Internet of Medical Things is growing rapidly in countries like China and India, driven by the increased focus on digital health and a growing number of connected devices.
- India's digital initiatives, such as the National Program for Digital India, aim to improve the delivery of health care through the use of IoT devices.
- Wearable devices such as smartwatches and fitness trackers are increasingly popular and have led to a rise in self-care.
MEA
- The Ministry of Health and Prevention in the United Arab Emirates is keen to harness the potential of IoMT to improve the quality of medical care, with initiatives to increase the capacity of telemedicine.
- The Vision 2030 plan foresees substantial investments in the health sector, which is expected to drive the uptake of IoMT solutions in the region.
- The lack of proper infrastructure and the different regulatory environments in the different countries of the region are limiting the pace of IoT adoption.
Latin America
- In Latin America, Brazil is the country most advanced in the Internet of Medical Things. The government has undertaken a number of projects aimed at modernizing the country’s health care system and increasing access to digital health solutions.
- In Mexico and Argentina, telemedicine is becoming popular, mainly because of the need for remote medical solutions in the face of the pandemic.
- IoMT has a strong presence in India, with a number of local start-ups that are working to address the unique health challenges of the country.
Did You Know?
“IoMT has been successfully implemented in about 30 per cent of hospitals in North America, with the aim of improving the quality of patient monitoring and the efficiency of care.” — Healthcare Information and Management Systems Society (HIMSS)
Segmental Market Size
The Internet of Medical Things (IoMT) is a crucial part of the IoT, as it enables the interconnection of medical devices and applications. The IoMT is currently growing strongly, driven by the growing demand for remote patient monitoring and telehealth services. The main drivers are the rising prevalence of chronic diseases and the need for effective healthcare management, especially in view of recent health crises.
IoMT is now in a scaled deployment phase, with leaders such as Philips and Medtronic implementing advanced IoMT solutions in different healthcare settings. The most important applications are remote monitoring of patients with chronic diseases, medication adherence systems and smart hospital environments. A pandemic such as COVID19 will further accelerate the spread of IoMT, as it reduces the need for in-person visits. Artificial intelligence and machine learning will also have a major impact on the evolution of this market, enabling more advanced data analytics and prescriptive health solutions.
Future Outlook
From 2023 to 2032, the IoMT market is expected to grow from a value of $ 63.25 billion to $156 billion, at a CAGR of 10.58 percent. The growth will be driven by the increasing use of connected medical devices, the development of telehealth and the increasing demand for remote patient monitoring solutions. By 2032, it is expected that over 50 percent of health care institutions will use IoMT solutions, which will improve patient care and increase the efficiency of health care.
In the same way, the key technological drivers of artificial intelligence, machine learning, and big data are expected to play a pivotal role in the evolution of the IoMT landscape. These technological developments will allow for more personalised health care, better prediction of patient outcomes, and improved data management. Moreover, the emergence of supportive government policies and regulations will facilitate the growth of the IoMT. The rise of wearable devices and smart medical devices, which will make health care more accessible and efficient, will also have a positive impact on the IoMT landscape.
Covered Aspects:| Report Attribute/Metric | Details |
|---|---|
| Growth Rate | 23.8% |
Internet Medical Things Market Highlights:
Leading companies partner with us for data-driven Insights
Kindly complete the form below to receive a free sample of this Report
Tailored for You
- Dedicated Research on any specifics segment or region.
- Focused Research on specific players in the market.
- Custom Report based only on your requirements.
- Flexibility to add or subtract any chapter in the study.
- Historic data from 2014 and forecasts outlook till 2040.
- Flexibility of providing data/insights in formats (PDF, PPT, Excel).
- Provide cross segmentation in applicable scenario/markets.









