Top Industry Leaders in the Ingestible Sensor Market

The Competitive Landscape of Ingestible Sensors:
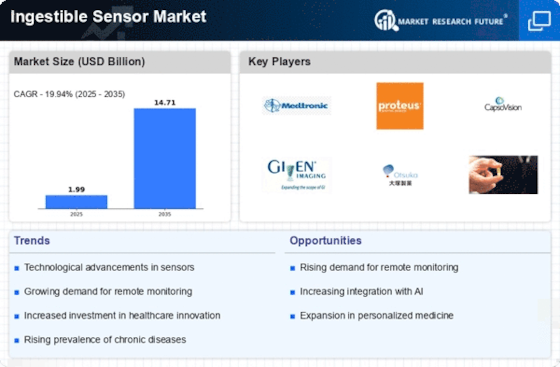
The ingestible sensor market, once a futuristic concept, is now hurtling towards mainstream adoption. These tiny, data-gathering capsules swallowed like pills are revolutionizing healthcare by offering real-time insights from within the digestive tract. This burgeoning market promises a paradigm shift in disease diagnosis, drug delivery, and personalized medicine, attracting a diverse range of players vying for a piece of the pie.
Key Players:
- RF Co. Ltd.
- Proteus Digital Health
- CapsoVision Inc.
- Medtronic
- Olympus Corporation
- IntroMedic Co. Ltd.
- MC10
- Otsuka Holdings
- AdhereTech
- Microchips Biotech Inc.
Strategies Adopted by Key Players:
- Product Innovation: Differentiation is key. Companies are pushing the boundaries with multi-sensor capabilities, extended data transmission ranges, and biodegradable materials.
- Strategic Partnerships: Collaborations with pharmaceutical companies, medical device giants, and data analytics firms unlock new markets and accelerate product development.
- Geographical Expansion: Targeting emerging markets with growing healthcare awareness and rising disposable incomes presents significant opportunities.
- Regulatory Compliance: Navigating the complex regulatory landscape for medical devices is crucial for market access and commercial success.
Current Competitive Landscape:
The market is currently dominated by a handful of established players, each with unique strengths and specialties. Proteus Digital Health, a pioneer in the field, boasts the FDA-approved Proteus Discover System, capable of tracking medication adherence and collecting vital data. Given Imaging, another major player, focuses on ingestible cameras like PillCam, offering unparalleled visualization of the small intestine. Olympus, a medical device giant, brings its expertise in endoscopy to the table with the PowerSpiral system, aiding in colonoscopy procedures.
New entrants are also emerging, shaking up the established order. MC10, a start-up, focuses on developing microfluidic sensors for ingestible devices, promising greater data collection capabilities. Medimetrics Personalized Drug Delivery offers capsules that not only monitor but also deliver medication directly to the target site, enhancing drug efficacy and reducing side effects. These innovators are pushing the boundaries of technology and application, forcing established players to adapt and innovate.
Factors for Market Share Analysis:
Analyzing market share in this dynamic landscape requires a multifaceted approach. While revenue and unit sales are crucial metrics, factors like:
- Technological Innovation: Companies with cutting-edge sensor technology, data analysis capabilities, and user-friendly platforms will hold an edge.
- Regulatory Landscape: Navigating the complex regulatory environment for ingestible devices is critical. Players with strong regulatory compliance and approvals will have a market advantage.
- Target Applications: Focusing on specific applications like gastrointestinal disorders, chronic diseases, or drug delivery will cater to specific needs and generate market traction.
- Strategic Partnerships: Collaborations with pharmaceutical companies, medical institutions, and research organizations can accelerate development, expand reach, and build trust.
New and Emerging Companies:
The influx of new ventures is a testament to the market's potential. Companies like HQ, specializing in ingestible sensors for gut microbiome analysis, and Microchips Biotech, developing sensor capsules for agricultural applications, are pushing the boundaries beyond human healthcare. This diversity indicates a future where ingestible sensors will not only diagnose and treat, but also monitor environmental health and optimize agricultural processes.
Industry Developments
RF Co. Ltd.
- October 26, 2023: RF Co. Ltd. announces it has completed a successful pilot study testing its ingestible sensor for monitoring intestinal motility in patients with chronic idiopathic constipation. The study, conducted in collaboration with Osaka University, demonstrated the sensor's accuracy and reliability in tracking gut movement, paving the way for further clinical trials.
- December 15, 2023: RF Co. Ltd. secures $10 million in Series B funding to accelerate the development and commercialization of its ingestible sensor technology. The funding round was led by healthcare venture capital firm, BioVentures, and will be used to expand clinical trials and bring the product to market.
Proteus Digital Health
- November 9, 2023: Proteus Digital Health partners with pharmaceutical company, Gilead Sciences, to develop an ingestible sensor system for monitoring medication adherence in patients with chronic diseases. The partnership aims to leverage Proteus's sensor technology and Gilead's expertise in drug development to improve medication compliance and patient outcomes.
- January 8, 2024: Proteus Digital Health announces the publication of a new study in the Journal of the American Medical Association demonstrating the effectiveness of its ingestible sensor system in improving medication adherence for heart failure patients. The study showed a significant increase in medication adherence among patients using the sensor system compared to the control group.