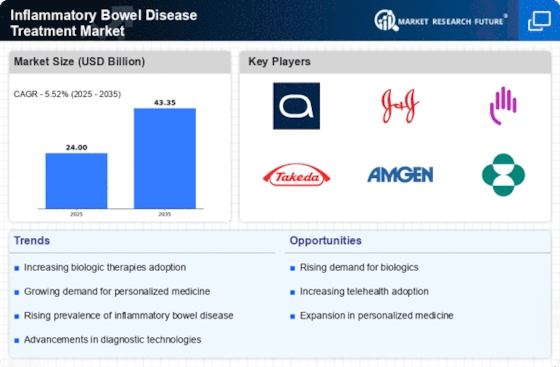
Top Industry Leaders in the Inflammatory Bowel Disease Treatment Market

Roche Holding AG Acquired Telavant, a company developing a promising anti-interleukin-23 (IL-23) antibody for IBD treatment, in a USD 7.1 billion deal. This positions Roche as a leader in developing IL-23 inhibitors for IBD.
AbbVie Received FDA approval for Rinvoq (upadacitinib), a JAK inhibitor, for the treatment of moderately to severely active ulcerative colitis. Rinvoq offers a new oral medication option for this patient population.
Takeda Pharmaceutical Company Limited Launched Entyvio (vedolizumab) in multiple new countries, expanding its reach for patients with Crohn's disease and ulcerative colitis.
Johnson & Johnson Received FDA approval for Stelara (ustekinumab) for the maintenance treatment of adults with moderately to severely active Crohn's disease. This further broadens Stelara's applications in IBD treatment.
Pfizer Partnered with Roche to develop next-generation antibody therapies for IBD. This collaboration combines expertise from both companies and could lead to innovative treatment options for IBD patients.
List of Inflammatory Bowel Disease Treatment Key Companies in the Market
- Abbott Laboratories
- Valeant Pharmaceuticals International
- Novartis AG
- Janssen Biotech, Inc.
- Alkem Laboratories Limited
- AbbVie, Inc.
- UCB Inc
- Takeda Pharmaceutical Company Limited
- Biogen Inc.
- Pfizer Inc.
- Allergan plc