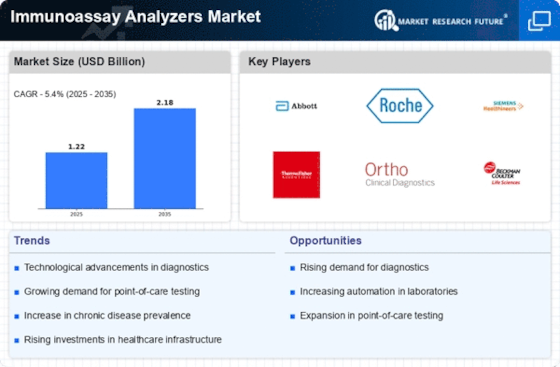
Top Industry Leaders in the Immunoassay Analyzers Market

Latest Immunoassay Analyzers Companies Updates:
Abbott Receives FDA Approval for Alinity h-series Hematology System:This November 2023 approval broadens Abbott's reach in the laboratory automation market, allowing labs to run complete blood counts (CBCs) on their Alinity h-series analyzers, streamlining workflows and enhancing efficiency.
Siemens Receives FDA Clearance for Atellica CI Analyzer:In July 2023, Siemens secured FDA clearance for its Atellica CI Analyzer, combining immunoassay and clinical chemistry capabilities in a single platform. This offers labs greater flexibility and reduces the need for multiple instruments.
DiaSorin Launches LIAISON B•R•A•H•M•S MR-proADM Assay:This CE-marked assay (December 2023) helps diagnose conditions like kidney diseases, sepsis, and septic shock, expanding DiaSorin's portfolio of immunoassay solutions for critical care applications.
Roche Signs Agreement with PerkinElmer for Automated Immunoassay Analyzer Development:This collaboration (October 2023) leverages PerkinElmer's expertise in automated liquid handling and miniaturization to develop next-generation immunoassay analyzers, potentially leading to more compact and versatile instruments.
Abbott and Quidel are actively developing miniaturized immunoassay analyzers for POCT settings, enabling faster diagnosis and treatment decisions closer to patients, particularly in emergency and critical care settings.
List of Immunoassay Analyzers Key companies in the market:
- Hoffmann-La Roche Ltd. (Switzerland)
- Siemens Ltd. (Germany)
- Abbott (US)
- Beckman Coulter Inc. (US)
- Ortho Clinical Diagnostics (US)
- bioMérieux Private Limited (France)
- Biokit S.A (Spain)
- The Binding Site Group Ltd. (UK)
- Immunodiagnostic Systems (UK)
- Merck KGaA (US)
- Olympus Corporation (Japan)
- Nova Century Scientific Inc. (US)
- Thermo Fisher Scientific (US)
- DiaSorin S.p.A. (Italy)