-
Definition
-
Scope of the Study
- Research Objective
- Assumptions
- Limitations
-
Introduction
-
Primary Research
-
Secondary Research
-
Market Size Estimation
-
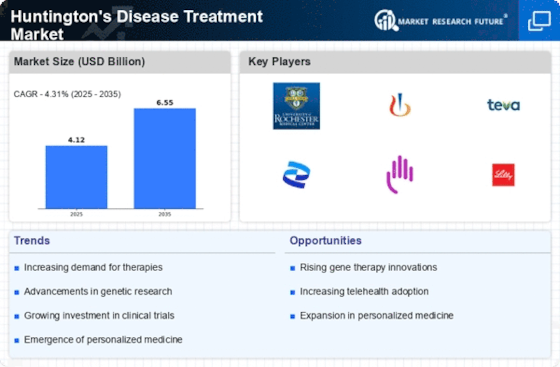
Drivers
-
Restraints
-
Opportunities
-
Challenges
-
Macroeconomic Indicators
-
Technology Trends & Assessment
-
Porter’s Five Forces Analysis
- Bargaining Power of Suppliers
- Bargaining Power of Buyers
- Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
Value Chain Analysis
-
Investment Feasibility Analysis
-
Pricing Analysis
-
Chapter 6. Global Huntington’Huntington’s Disease Treatment Market, by Drug Type
-
Introduction
-
Tetrabenazine
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Deutetrabenazine
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Selective Serotonin Reuptake Inhibitor (SSRI)
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Chlorpromazine
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Haloperidol
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Risperidone
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Olanzapine
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Clozapine
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Other
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Chapter 7. Global Huntington’Huntington’s Disease Treatment Market, by Treatment
-
Introduction
-
Symptomatic therapy
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Disease-Modifying therapy
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Chapter 8. Global Huntington’Huntington’s Disease Treatment Market, by End-Users
-
Introduction
-
Hospital
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Clinics
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Retail Pharmacies
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Online Pharmacies
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Others
-
Market Estimates & Huntington’s Disease Treatment Market, by Region, 2020–2027
-
Market Estimates & Huntington’s Disease Treatment Market, by Country, 2020–2027
-
Chapter 9. Global Huntington’Huntington’s Disease Treatment Market, by Region
-
Introduction
-
America
- North America
- South America
-
Europe
- Western Europe
- Eastern Europe
-
Asia-Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia-Pacific
-
Middle East & Africa
- Middle East
- Africa
-
Chapter 10. Company Landscape
-
Introduction
-
Market Share Analysis
-
Key Development & Strategies
-
Chapter 11. Company Profiles
-
Pfizer, Inc.
- Company Overview
- Product Overview
- Financials Overview
- Key Developments
- SWOT Analysis
-
Alnylam Pharmaceuticals Inc
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
-
AmpliPhi Biosciences Corp
- Company Overview
- Product Overview
- Financial Overview
- Key Development
- SWOT Analysis
-
Ceregene Inc
- Company Overview
- Product Overview
- Financial Overview
- Key Development
- SWOT Analysis
-
Prana Biotechnology Limited
- Company Overview
- Product Overview
- Financial overview
- Key Developments
- SWOT Analysis
-
Teva Pharmaceutical Industries Ltd
- Company Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
-
Cortex Pharmaceuticals Inc
- Overview
- Product Overview
- Financial Overview
- Key Developments
- SWOT Analysis
-
Vertex Pharmaceuticals Incorporated
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Auspex Pharmaceuticals
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
SOM Biotech
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
GlaxoSmithKline
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Siena Biotech
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Raptor Pharmaceutical
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Palobiofarma
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Omeros
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Ipsen
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Valeant Pharmaceuticals International Inc
- Overview
- Product Overview
- Financials
- Key Developments
- SWOT Analysis
-
Chapter 12 MRFR Conclusion
-
Key Findings
- From CEO’s Viewpoint
- Unmet Needs of the Market
-
Key Companies to Watch
-
Predictions for the Global Huntington’s Disease Treatment Market
-
Chapter 13. Appendix
-
-
LIST OF TABLES
-
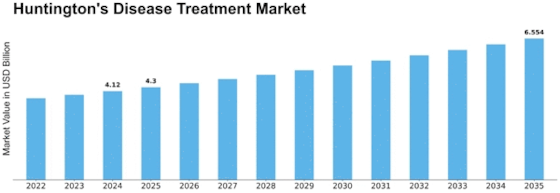
Global Huntington’s Disease Treatment Market Synopsis, 2020-2027
-
Global Huntington’s Disease Treatment Market Estimates and Forecast, 2020-2027 (USD Million)
-
Global Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
Global Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
Global Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
Global Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027(USD Million)
-
North America: Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
North America: Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
North America: Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
North America: Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027 (USD Million)
-
US: Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
US: Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
US: Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
US: Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027 (USD Million)
-
Canada: Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
Canada: Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
Canada: Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
Canada: Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027 (USD Million)
-
South America: Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
South America: Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
South America: Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
South America: Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027 (USD Million)
-
Europe: Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
Europe: Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
Europe: Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
Europe: Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027 (USD Million)
-
Western Europe: Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
Western Europe: Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
Western Europe: Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
Western Europe: Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027 (USD Million)
-
Eastern Europe: Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
Eastern Europe: Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
Eastern Europe: Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
Eastern Europe: Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027 (USD Million)
-
Asia-Pacific: Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
Asia-Pacific: Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
Asia-Pacific: Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
Asia-Pacific: Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027 (USD Million)
-
Middle East & Africa: Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020-2027 (USD Million)
-
Middle East & Africa: Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020-2027 (USD Million)
-
Middle East & Africa: Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020-2027 (USD Million)
-
Middle East & Africa: Huntington’Huntington’s Disease Treatment Market, by Region, 2020-2027 (USD Million)
-
LIST OF FIGURES
-
Research Process
-
Segmentation for Global Huntington’s Disease Treatment Market
-
Segmentation Market Dynamics for Global Huntington’s Disease Treatment Market
-
Global Huntington’Huntington’s Disease Treatment Market, by Drug Type, 2020(%)
-
Global Huntington’Huntington’s Disease Treatment Market, by Treatment, 2020(%)
-
Global Huntington’Huntington’s Disease Treatment Market, by End-Users, 2020(%)
-
Global Huntington’Huntington’s Disease Treatment Market, by Region, 2020(%)
-
North America: Huntington’Huntington’s Disease Treatment Market, by Country, 2020(%)
-
Europe: Huntington’Huntington’s Disease Treatment Market, by Country, 2020(%)
-
Asia-Pacific: Huntington’Huntington’s Disease Treatment Market, by Country, 2020(%)
-
Middle East & Africa: Huntington’Huntington’s Disease Treatment Market, by Country,2020(%)
-
Global Huntington’s Disease Treatment Market: Company Share Analysis, 2020(%)
-
Pfizer, Inc: Key Financials
-
Pfizer, Inc: Segmental Revenue
-
Pfizer, Inc: Geographical Revenue
-
Alnylam Pharmaceuticals Inc: Key Financials
-
Alnylam Pharmaceuticals Inc: Segmental Revenue
-
Alnylam Pharmaceuticals Inc: Geographical Revenue
-
AmpliPhi Biosciences Corp: Key Financials
-
AmpliPhi Biosciences Corp: Segmental Revenue
-
AmpliPhi Biosciences Corp: Geographical Revenue
-
Ceregene Inc: Key Financials
-
Ceregene Inc: Segmental Revenue
-
Ceregene Inc: Geographical Revenue
-
Prana Biotechnology Limited.: Key Financials
-
Prana Biotechnology Limited.: Segmental Revenue
-
Prana Biotechnology Limited.: Geographical Revenue
-
Teva Pharmaceutical Industries Ltd.: Key Financials
-
Teva Pharmaceutical Industries Ltd.: Segmental Revenue
-
Teva Pharmaceutical Industries Ltd.: Geographical Revenue
-
Cortex Pharmaceuticals Inc: Key Financials
-
Cortex Pharmaceuticals Inc: Segmental Revenue
-
Cortex Pharmaceuticals Inc: Geographical Revenue
-
Vertex Pharmaceuticals Incorporated: Key Financials
-
Vertex Pharmaceuticals Incorporated: Segmental Revenue
-
Vertex Pharmaceuticals Incorporated: Geographical Revenue
-
Auspex Pharmaceuticals: Key Financials
-
Auspex Pharmaceuticals: Segmental Revenue
-
Auspex Pharmaceuticals: Geographical Revenue
-
SOM Biotech: Key Financials
-
SOM Biotech: Segmental Revenue
-
SOM Biotech: Geographical Revenue
-
GlaxoSmithKline: Key Financials
-
GlaxoSmithKline: Segmental Revenue
-
GlaxoSmithKline: Geographical Revenue
-
Siena Biotech: Key Financials
-
Siena Biotech: Segmental Revenue
-
Siena Biotech: Geographical Revenue
-
Raptor Pharmaceutical.: Key Financials
-
Raptor Pharmaceutical.: Segmental Revenue
-
Raptor Pharmaceutical.: Geographical Revenue
-
Palobiofarma: Key Financials
-
Palobiofarma: Segmental Revenue
-
Palobiofarma: Geographical Revenue
-
Omeros: Key Financials
-
Omeros: Segmental Revenue
-
Omeros: Geographical Revenue
-
Ipsen: Key Financials
-
Ipsen: Segmental Revenue
-
Ipsen: Geographical Revenue
-
Valeant Pharmaceuticals International Inc: Key Financials
-
Valeant Pharmaceuticals International Inc: Segmental Revenue
-
Valeant Pharmaceuticals International Inc: Geographical Revenue

















Leave a Comment