Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia A
Hemophilia B
Von Willebrand Disease
Intravenous
Subcutaneous
Oral
Hospitals
Specialty Clinics
Home Care Settings
North America
Europe
South America
Asia Pacific
Middle East and Africa
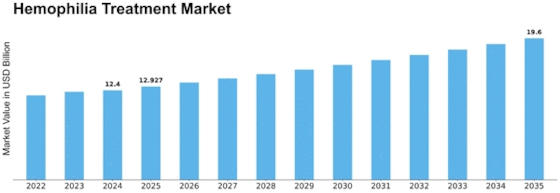
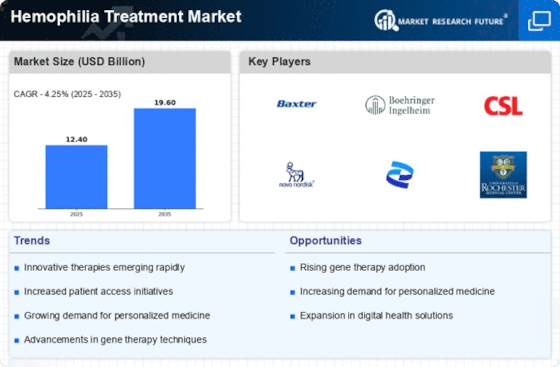
North America Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
Hemophilia Treatment Market by Regional Type
US
Canada
US Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
CANADA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
Europe Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
Hemophilia Treatment Market by Regional Type
Germany
UK
France
Russia
Italy
Spain
Rest of Europe
GERMANY Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
UK Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
FRANCE Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
RUSSIA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
ITALY Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
SPAIN Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
REST OF EUROPE Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
APAC Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
Hemophilia Treatment Market by Regional Type
China
India
Japan
South Korea
Malaysia
Thailand
Indonesia
Rest of APAC
CHINA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
INDIA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
JAPAN Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
SOUTH KOREA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
MALAYSIA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
THAILAND Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
INDONESIA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
REST OF APAC Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
South America Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
Hemophilia Treatment Market by Regional Type
Brazil
Mexico
Argentina
Rest of South America
BRAZIL Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
MEXICO Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
ARGENTINA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
REST OF SOUTH AMERICA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
MEA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
Hemophilia Treatment Market by Regional Type
GCC Countries
South Africa
Rest of MEA
GCC COUNTRIES Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
SOUTH AFRICA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings
REST OF MEA Outlook (USD Billion, 2019-2035)
Hemophilia Treatment Market by Treatment Type
Prophylactic Treatment
On-Demand Treatment
Gene Therapy
Desmopressin
Factor Replacement Therapy
Hemophilia Treatment Market by Hemophilia Type
Hemophilia A
Hemophilia B
Von Willebrand Disease
Hemophilia Treatment Market by Administration Route Type
Intravenous
Subcutaneous
Oral
Hemophilia Treatment Market by End User Type
Hospitals
Specialty Clinics
Home Care Settings

















Leave a Comment