Gross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Kidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Drugs
Therapeutics
Others
Hospitals
Clinics
Laboratories
Research Centers
Others
North America Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
North America Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
North America Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
North America Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
US Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
US Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
US Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
US Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
CANADA Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
CANADA Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
CANADA Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
CANADA Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Europe Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Europe Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Europe Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Europe Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Germany Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Germany Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Germany Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Germany Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
France Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
France Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
France Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
France Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
UK Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
UK Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
UK Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
UK Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
ITALY Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
ITALY Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
ITALY Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
ITALY Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Spain Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Spain Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Spain Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Spain Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Rest Of Europe Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
REST OF EUROPE Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
REST OF EUROPE Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
REST OF EUROPE Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Asia-Pacific Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Asia-Pacific Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Asia-Pacific Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Asia-Pacific Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
China Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
China Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
China Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
China Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Japan Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Japan Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Japan Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Japan Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
India Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
India Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
India Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
India Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Australia Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Australia Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Australia Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Australia Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Rest of Asia-Pacific Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Rest of Asia-Pacific Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Rest of Asia-Pacific Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Rest of Asia-Pacific Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Rest of the World Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Rest of the World Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Rest of the World Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Rest of the World Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Middle East Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Middle East Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Middle East Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Middle East Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Africa Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Africa Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Africa Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Africa Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
Latin America Hematuria Treatment by TypeGross Or Macroscopic Hematuria
Microscopic Hematuria
Idiopathic Hematuria
Jogger's Hematuria
Latin America Hematuria Treatment by CausesKidney Stones
Urinary Tract Infections (UTI)
Urethritis
Blood Cancer
Bladder Stones
Prostate Cancer
Cystitis
Trauma
Vigorous Exercise
Polycystic Kidney Disease
Endometriosis
Menstruation
Latin America Hematuria Treatment by TreatmentDrugs
Therapeutics
Others
Latin America Hematuria Treatment by End-UsersHospitals
Clinics
Laboratories
Research Centers
Others
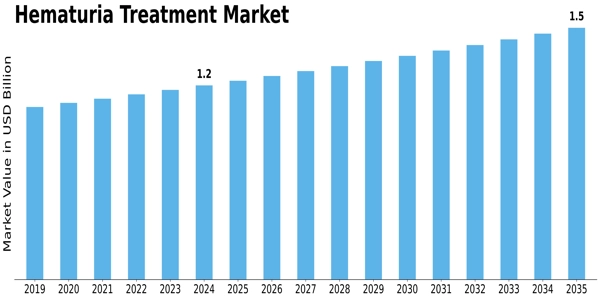

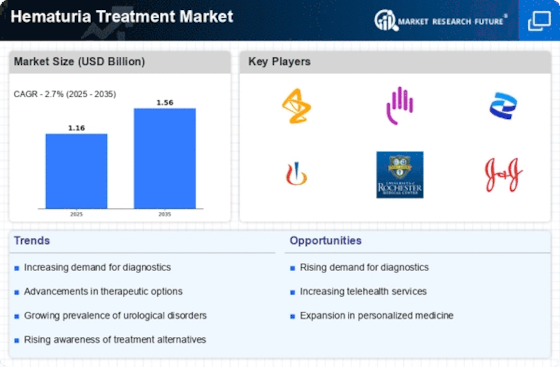







Leave a Comment