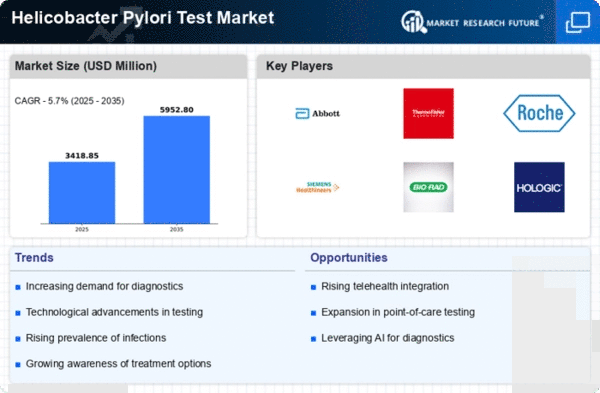
Top Industry Leaders in the Helicobacter Pylori Test Market

Latest H. Pylori Test Companies Update:
Biomerica received US FDA 510(k) clearance for its Hp Detect™ ELISA Test, a new non-invasive stool antigen test for detecting H. pylori infection. This clearance opens up a significant market opportunity for Biomerica in the US, where an estimated 35% of the population is infected with H. pylori.
Meridian Bioscience announced the launch of its Meridian HpFAST® Urea Breath Test in Brazil, expanding its reach in the Latin American market for H. pylori testing. This test offers a quick and accurate means of diagnosing H. pylori infection.
Roche Diagnostics introduced the Elecsys® Anti- Helicobacter pylori IgG assay, a new automated immunoassay for serological detection of H. pylori infection. This assay offers high sensitivity and specificity, improving diagnostic accuracy.
Quidel Corporation launched the Sofia® H. pylori Antigen FIA test, a rapid point-of-care test for detecting H. pylori infection in stool samples. This test provides results in just 15 minutes, facilitating faster diagnosis and treatment decisions.
List of H. Pylori Test Key companies in the market
-
Thermo Fisher Scientific Inc. (US),
-
Bio-Rad Laboratories, Inc. (US),
-
Hoffmann-La Roche Ltd (Switzerland),
-
Quest Diagnostics Incorporated (US),
-
Meridian Bioscience (US),
-
CorisBioconcept SPRL (Belgium),
-
Cardinal Health (US),
-
QIAGEN (Germany),
-
Abbott Laboratories (US),
-
Biohit Oyj (Finland)