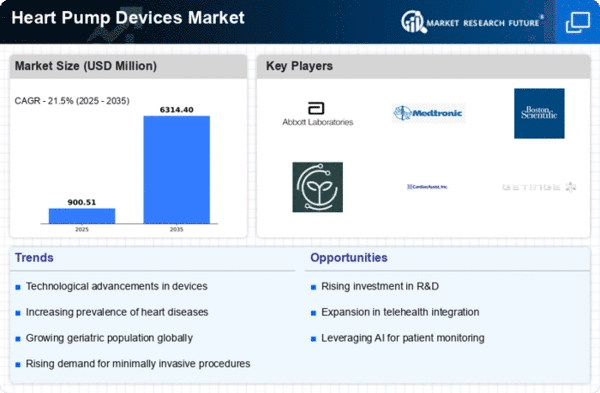
Market Trends
Key Emerging Trends in the Heart Pump Devices Market
In the recent years the market for heart pumps has been really in trend and this is due to the advancements that have been made in medical science and the higher prevalence of the diseases of the heart. The surge in VADs uptake presents one of the major recent trends as an alternative to surgical transplantation. These devices been known to be the most effective devices that offer the quality of life for patients waiting to be included in the list in for heart transplantation or those ineligible for transplantation. The market nowadays witnesses a growth in the demand of both intracorporeal and extra corporeal ventricle assist devices and giving users, the flexibility to choose among available options for their medical needs.
Technological innovations are critically important in the evolution of market patterns, in where the continuous efforts being made to enhance device performance and minimized invasiveness are constantly being pursued and improved. The requirement for the miniaturization of heart pumps has now become very demanding, which in turn leads to smaller devices, and therefore, procedures with little or no injury to patients and lower risk of complications. Many new materials and better designs have improved the life span of pumps by making them more durable and dependable. Thus, the heart pumps become more efficient and people live longer. Therefore, healthcare professionals are now incorporating technologies in an increased rate to enable them to offer more innovative treatments for patients who are suffering from heart failure that is already advanced.
A new trend (the destination therapy) in the implantable heart devices market is the given long term solution for patients with no transplant options to follow. VADs (ventricular assist devices) as destination therapy it always enables the patients to have active and improved living with their cardiac problems. This situation indicates the shift in the attitude of a ventricular assist devices from an emergency solution for a short period of time to a permanent one, liberating patients who might have otherwise no other choices.
Additionally, a collaboration and partnership between the vital participants in the industry would be seen as the major market trend recently. This co-operative approach intends to mobilize the accumulated competitiveness as well as the resources of the different stakeholders, for example, those working in the area of medical devices, healthcare providers and the scientific institutions. Collaboration, which is seen as a key driver, allows the cardiovascular industry and other stakeholders to develop the solutions that address the diverse and systemic problems related to cardiovascular diseases and thus deliver better treatment outcomes for patients.
Another market trend which is worth noting is the customer experience that consists of heightened awareness and use of digital means for the management of patients powered with heart pumps. These mechanics therefore correspond with the general movement in the sphere of digital health and patient-oriented care, and contribute to improvement of the situation in terms of patient experience and their adherence to therapies.
















Leave a Comment