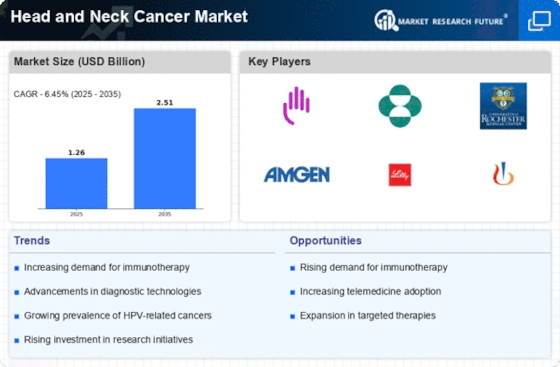
Top Industry Leaders in the Head and Neck Cancer Market

Latest Head and Neck Cancer Companies Updates:
Merck & Co.'s Keytruda (pembrolizumab) Approval: This immunotherapy drug received FDA approval for the first-line treatment of recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) with PD-L1 expression, offering a promising new option for patients.
Bristol Myers Squibb's Opdivo (nivolumab) Combo Therapy: This drug, in combination with cetuximab, demonstrated promising results in a Phase 3 trial for treating recurrent or metastatic HNSCC, potentially paving the way for more effective combination therapies.
AstraZeneca's Lynparza (olaparib) for BRCA Mutations: This drug received FDA approval for the maintenance treatment of adult patients with locally advanced or metastatic HNSCC harboring BRCA gene mutations, offering a targeted therapy option for a specific patient subgroup.
Personalis, Inc.'s NGS Companion Diagnostic: This company's next-generation sequencing (NGS) test was granted FDA approval as a companion diagnostic for Lynparza, helping identify patients who may benefit most from this targeted therapy.
List of Head and Neck Cancer Key companies in the market:
- Bayer Healthcare AG
- Boston Biomedical, Inc.
- Bristol-Myers Squibb Company
- Astellas Pharma Inc.
- AbbVie Inc.
- AstraZeneca Plc.
- Acceleron Pharma Inc.
- DentalEZ, Inc.
- Koninklijke Philips N.V.
- Siemens Healthcare Private Limited
- AB Sciences