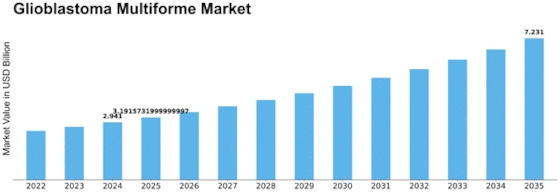
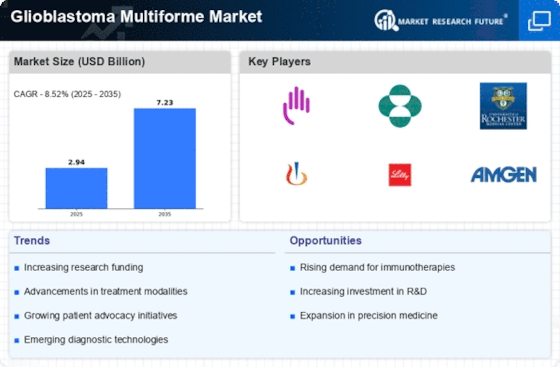
Glioblastoma Multiforme Size
Glioblastoma Multiforme Market Growth Projections and Opportunities
There are several elements that affect the Glioblastoma Multiforme (GBM) market, which together determine its dynamics. The frequency of GBM, a very aggressive and deadly kind of brain cancer, is one of the main causes. Research and development activities in the market are being driven by the growing need for effective therapies as the prevalence of GBM grows internationally. Innovations in cancer research and therapy have a significant impact on the GBM market. Ongoing advancements in targeted treatments, genetics, and imaging methods help us understand the biology of GBM better and provide more individualized treatment plans. Technological developments in radiation treatment and neurosurgery have an effect on GBM care as a whole. One important component affecting market dynamics is the regulatory environment around cancer medicines. Strict regulatory guidelines guarantee the efficacy, safety, and caliber of GBM therapies, which affects their acceptance, marketability, and availability. Gaining market acceptance and establishing confidence with patients, regulatory bodies, and healthcare providers depend on compliance with these regulations. variable areas have variable rates of GBM therapy uptake based on healthcare facilities and economic reasons. The demand for relevant treatment choices and the prevalence of GBM therapies are influenced by changes in healthcare access, reimbursement regulations, and economic situations. In order to guarantee that their GBM therapies meet the particular difficulties presented by various healthcare systems, market participants must traverse these heterogeneous environments. One important factor in the GBM market is the patient-centric approach to cancer care. Treatments that put patient outcomes, quality of life, and a decrease in adverse effects from therapy first are becoming more and more popular. The patient-centered paradigm in contemporary healthcare is reflected in the development of GBM therapies that improve the whole patient experience and adhere to the principles of individualized medicine. Two new variables that are affecting the GBM market are cost-effectiveness and market accessibility. The optimization of resource use and the reduction of the total economic burden of cancer care present difficulties to healthcare systems globally. Treatments for GBM that have higher efficacy, less adverse effects, and more economical results become desirable choices for payers and healthcare providers alike.

















Leave a Comment