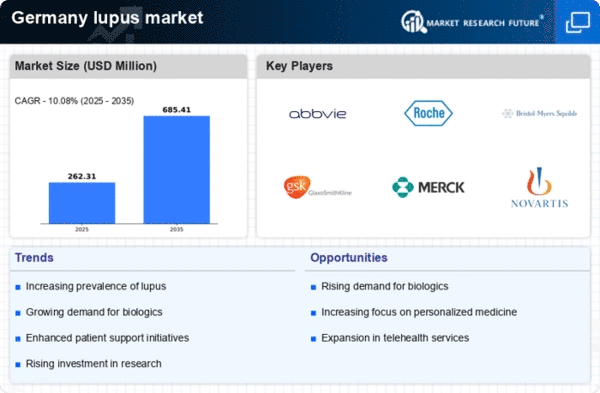
Increasing Prevalence of Lupus
The rising incidence of lupus in Germany is a critical driver for the lupus market. Recent studies indicate that the prevalence of lupus has increased by approximately 20% over the last decade. This growing patient population necessitates enhanced healthcare services and treatment options, thereby expanding the market. The lupus market is likely to experience significant growth as more individuals are diagnosed and seek effective management strategies. Furthermore, the increasing awareness among healthcare professionals about lupus symptoms and complications contributes to earlier diagnosis, which may lead to a higher demand for therapeutic interventions. As the number of diagnosed cases rises, pharmaceutical companies are incentivized to invest in research and development, potentially leading to innovative therapies that cater to the specific needs of the German population.
Enhanced Patient Support Programs
The establishment of comprehensive patient support programs is emerging as a vital driver for the lupus market in Germany. These programs aim to provide patients with resources, education, and emotional support, which can significantly improve treatment adherence and overall quality of life. Organizations dedicated to lupus advocacy are increasingly collaborating with healthcare providers to create awareness and facilitate access to necessary treatments. In 2025, it is estimated that over 30% of lupus patients in Germany will benefit from such support initiatives. By addressing the psychosocial aspects of living with lupus, these programs may enhance patient engagement and satisfaction, ultimately leading to better health outcomes. The positive impact of patient support initiatives is likely to contribute to the growth of the lupus market as more individuals seek out these valuable resources.
Regulatory Support for New Therapies
The regulatory landscape in Germany is becoming increasingly supportive of new therapies for lupus, which serves as a significant driver for the lupus market. The German Medicines Agency has streamlined the approval process for innovative treatments, allowing for faster access to new medications. This regulatory flexibility is particularly beneficial for patients with unmet medical needs, as it encourages pharmaceutical companies to invest in the development of novel therapies. In 2025, it is anticipated that the approval rate for new lupus treatments will increase by approximately 15%, reflecting the proactive stance of regulatory bodies. As new therapies enter the market, competition is likely to intensify, leading to improved treatment options for patients. This supportive regulatory environment is expected to foster growth in the lupus market, ultimately benefiting both patients and healthcare providers.
Advancements in Diagnostic Technologies
Technological advancements in diagnostic tools are transforming the lupus market in Germany. Enhanced imaging techniques and biomarker identification have improved the accuracy and speed of lupus diagnosis. For instance, the introduction of advanced blood tests can detect specific autoantibodies associated with lupus, facilitating timely treatment initiation. This shift towards precision medicine is likely to increase patient engagement and adherence to treatment regimens. As diagnostic capabilities improve, healthcare providers can better stratify patients based on disease severity, which may lead to more personalized treatment approaches. Consequently, the demand for innovative diagnostic solutions is expected to rise, further propelling the lupus market. The integration of these technologies into clinical practice may also enhance patient outcomes, thereby fostering a more robust market environment.
Growing Investment in Research and Development
Investment in research and development (R&D) is a pivotal driver for the lupus market in Germany. Pharmaceutical companies are increasingly allocating resources to discover novel therapies and improve existing treatment options for lupus. In 2025, R&D spending in the biopharmaceutical sector is projected to reach €10 billion, with a significant portion directed towards autoimmune diseases like lupus. This influx of funding is likely to accelerate the development of targeted therapies and biologics, which may offer improved efficacy and safety profiles. Additionally, collaborations between academic institutions and industry players are fostering innovation, potentially leading to breakthroughs in lupus treatment. As the pipeline for new therapies expands, the lupus market is expected to grow, providing patients with more effective management options.