Enhanced Patient Support Programs
The establishment of comprehensive patient support programs is emerging as a vital driver for the epilepsy market in Germany. These programs aim to provide education, resources, and emotional support to individuals living with epilepsy and their families. By fostering a better understanding of the condition, these initiatives can improve treatment adherence and overall patient satisfaction. In recent years, several organizations have launched campaigns to raise awareness and provide resources, which has positively influenced the market. As patient-centric care becomes increasingly prioritized, the epilepsy market is likely to see growth in services that cater to the holistic needs of patients, ultimately enhancing their quality of life.
Increasing Prevalence of Epilepsy
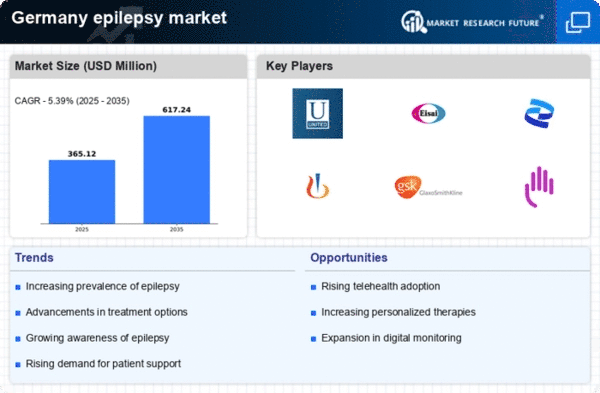
The rising incidence of epilepsy in Germany is a crucial driver for the epilepsy market. Recent estimates indicate that approximately 600,000 individuals are living with epilepsy in the country, which translates to a prevalence rate of about 0.8% of the population. This growing patient base necessitates enhanced treatment options and healthcare services, thereby stimulating demand within the epilepsy market. Furthermore, the increasing awareness and diagnosis of epilepsy contribute to this trend, as more individuals seek medical attention for their symptoms. As healthcare providers become more adept at identifying epilepsy, the market is likely to expand, leading to a greater focus on innovative therapies and management strategies.
Advancements in Diagnostic Technologies
Technological innovations in diagnostic tools are significantly impacting the epilepsy market in Germany. The introduction of advanced imaging techniques, such as MRI and EEG, has improved the accuracy of epilepsy diagnoses. These advancements enable healthcare professionals to better understand the underlying causes of seizures, leading to more effective treatment plans. The market for diagnostic devices is projected to grow, with estimates suggesting a compound annual growth rate (CAGR) of around 7% over the next five years. This growth is driven by the increasing demand for precise and timely diagnoses, which is essential for effective management of epilepsy. As diagnostic technologies continue to evolve, they are expected to play a pivotal role in shaping the future of the epilepsy market.
Regulatory Framework for Drug Approvals
The regulatory environment surrounding drug approvals is a critical factor influencing the epilepsy market in Germany. The European Medicines Agency (EMA) and the Federal Institute for Drugs and Medical Devices (BfArM) play essential roles in ensuring the safety and efficacy of new treatments. Recent initiatives aimed at expediting the approval process for innovative therapies are likely to enhance market dynamics. For instance, the introduction of adaptive pathways allows for more flexible regulatory approaches, potentially reducing time to market for new epilepsy medications. This supportive regulatory framework may encourage pharmaceutical companies to invest in the development of novel treatments, thereby driving growth in the epilepsy market.
Growing Investment in Research and Development
Investment in research and development (R&D) is a significant driver for the epilepsy market in Germany. Pharmaceutical companies and research institutions are increasingly focusing on developing novel antiepileptic drugs and therapies. In 2025, R&D spending in the neurology sector is projected to reach approximately €1.5 billion, reflecting a commitment to addressing unmet medical needs in epilepsy treatment. This influx of funding is likely to accelerate the discovery of innovative therapies, including biologics and gene therapies, which may offer new hope for patients. As the landscape of epilepsy treatment evolves, the epilepsy market is expected to benefit from these advancements, leading to improved patient outcomes and quality of life.