Advancements in Genetic Research
Ongoing advancements in genetic research are significantly influencing the dravet syndrome market. The identification of specific genetic mutations associated with Dravet syndrome has paved the way for targeted therapies. In Germany, research institutions are increasingly focusing on gene therapy and precision medicine, which may offer more effective treatment options. This shift towards personalized medicine is likely to enhance patient outcomes and drive market growth. The dravet syndrome market stands to benefit from these innovations, as they may lead to the development of new drugs and treatment protocols tailored to individual genetic profiles.
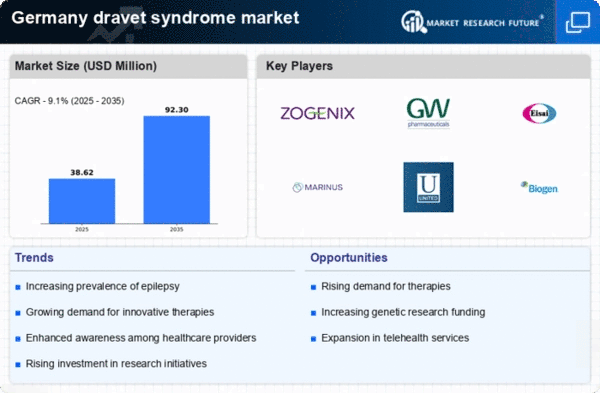
Increasing Prevalence of Dravet Syndrome
The rising incidence of Dravet syndrome in Germany is a crucial driver for the dravet syndrome market. Recent studies indicate that the prevalence of this rare genetic epilepsy disorder is approximately 1 in 15,700 live births. This growing number of diagnosed cases necessitates enhanced treatment options and healthcare resources, thereby expanding the market. As awareness increases among healthcare professionals and families, more patients are likely to seek specialized care, which could lead to a surge in demand for effective therapies. The dravet syndrome market is thus positioned to grow as healthcare systems adapt to meet the needs of this expanding patient population.
Government Support and Funding Initiatives
Government initiatives aimed at supporting rare disease research are vital for the dravet syndrome market. In Germany, funding programs and grants for research into rare diseases have increased, providing financial resources for clinical trials and innovative treatment development. This support not only encourages pharmaceutical companies to invest in the dravet syndrome market but also fosters collaboration between academia and industry. As a result, the market is likely to see a rise in new therapies and treatment options, enhancing the overall landscape for patients and healthcare providers.
Growing Patient Advocacy and Support Groups
The emergence of patient advocacy groups in Germany plays a significant role in shaping the dravet syndrome market. These organizations raise awareness about the condition, promote research funding, and provide resources for families affected by Dravet syndrome. Their efforts contribute to a more informed public and healthcare community, which can lead to earlier diagnosis and treatment. As advocacy groups continue to grow, they may influence policy changes and healthcare practices, ultimately driving demand for therapies within the dravet syndrome market.
Integration of Telemedicine in Patient Care
The integration of telemedicine into patient care is transforming the dravet syndrome market in Germany. Telehealth services provide patients with easier access to specialists, particularly in rural areas where healthcare resources may be limited. This increased accessibility can lead to more timely diagnoses and treatment plans, which is crucial for managing Dravet syndrome effectively. As telemedicine continues to evolve, it is likely to enhance patient engagement and adherence to treatment protocols, thereby positively impacting the dravet syndrome market.