-
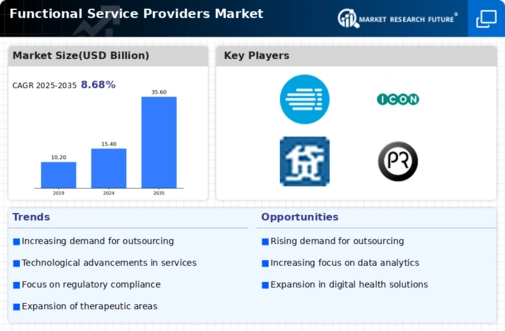
EXECUTIVE SUMMARY
-
MARKET INTRODUCTION
-
DEFINITION
-
SCOPE OF THE STUDY
-
RESEARCH OBJECTIVE
-
MARKET STRUCTURE
-
ASSUMPTIONS & LIMITATIONS
-
RESEARCH METHODOLOGY
-
DATA MINING
-
SECONDARY RESEARCH
-
PRIMARY RESEARCH
-
BREAKDOWN OF PRIMARY RESPONDENTS
-
FORECASTING TECHNIQUES
-
RESEARCH METHODOLOGY FOR MARKET SIZE ESTIMATION
- BOTTOM-UP APPROACH
- TOP-DOWN APPROACH
-
DATA TRIANGULATION
-
VALIDATION
-
MARKET DYNAMICS
-
OVERVIEW
-
DRIVERS
- INCREASING R&D INVESTMENTS
- RISING NUMBER OF CLINICAL TRIALS
- LOWERED OPERATIONAL COSTS AND IMPROVED SERVICE QUALITY
- RISE IN FSP OUTSOURCING IN DRUG DEVELOPMENT
-
RESTRAINTS
- DEARTH OF SKILLED LABOR
-
OPPORTUNITIES
- BIOPHARMA AND BIOTECH’S DIGITAL TRANSFORMATION
-
MARKET FACTOR ANALYSIS
-
VALUE CHAIN ANALYSIS
- CUSTOMER ACQUISITION (PREPURCHASE QUERY)
- SERVICE CUSTOMIZATION
- CUSTOMER PURCHASE SUPPORT
- CUSTOMER FULFILMENT & POST-PURCHASE SUPPORT
-
PORTER''S FIVE FORCES MODEL
- THREAT OF NEW ENTRANTS
- BARGAINING POWER OF SUPPLIERS
- THREAT OF SUBSTITUTES
- BARGAINING POWER OF BUYERS
- INTENSITY OF RIVALRY
-
IMPACT OF COVID-19 ON FUNCTIONAL SERVICE PROVIDERS MARKET
- OVERVIEW
- IMPACT ON DEMAND FOR FUNCTIONAL SERVICE PROVIDERS (FSP)
-
GLOBAL FUNCTIONAL SERVICE PROVIDERS MARKET BY TYPE
-
OVERVIEW
-
CLINICAL MONITORING
-
MEDICAL WRITING
-
DATA MANAGEMENT
-
PHARMACOVIGILANCE
-
BIOSTATISTICS
-
PROGRAMMING
-
STUDY DESIGN
-
OTHERS
-
GLOBAL FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE
-
OVERVIEW
-
CLINICAL DEVELOPMENT
-
POST APPROVAL
-
GLOBAL FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION
-
OVERVIEW
-
BIOPHARMA COMPANIES
-
BIOTECH COMPANIES
-
MEDICAL DEVICES COMPANIES
-
RESEARCH CENTER AND ACADEMIC INSTITUTES
-
Functional Service Providers (FSP) Market, BY REGION
-
GLOBAL
-
NORTH AMERICA
- US
- CANADA
-
EUROPE
- GERMANY
- FRANCE
- UK
- ITALY
- SPAIN
- REST OF EUROPE
-
ASIA-PACIFIC
- CHINA
- JAPAN
- INDIA
- AUSTRALIA
- SOUTH KOREA
- REST OF ASIA-PACIFIC
-
REST OF THE WORLD
- LATIN AMERICA
- MIDDLE EAST
- AFRICA
-
COMPETITIVE LANDSCAPE
-
OVERVIEW
-
COMPETITIVE BENCHMARKING
-
MAJOR GROWTH STRATEGY IN THE GLOBAL FUNCTIONAL SERVICE PROVIDERS MARKET
-
THE LEADING PLAYERS IN TERMS OF NUMBER OF DEVELOPMENTS IN THE GLOBAL FUNCTIONAL SERVICE PROVIDERS MARKET
-
KEY DEVELOPMENT ANALYSIS
-
KEY DEVELOPMENTS & GROWTH STRATEGIES
- PRODUCT LAUNCHES
- PARTNERSHIPS
- ACQUISITIONS
-
MAJOR PLAYERS FINANCIAL MATRIX
- SALES (USD BILLION), 2022
- RESEARCH & DEVELOPMENT EXPENDITURE (USD BILLION), 2022
-
COMPANY PROFILES
-
IQVIA INC.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- SOLUTIONS/SERVICES OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
LABORATORY CORPORATION OF AMERICA HOLDINGS
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
ICON PLC
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
KPS LIFE, LLC
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
WUXI APPTEC CO., LTD.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- SOLUTIONS/SERVICES OFFERED
- KEY DEVELOPMENTS
- SWOT ANALYSIS
- KEY STRATEGIES
-
PPD INC.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- SOLUTIONS/SERVICES OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
PAREXEL INTERNATIONAL CORPORATION
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
QUANTICATE INTERNATIONAL LIMITED
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
BIOPOINT INC.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
RHO, INC.
- COMPANY OVERVIEW
- FINANCIAL OVERVIEW
- PRODUCTS/SERVICES OFFERED
- KEY DEVELOPMENTS
- KEY STRATEGIES
-
APPENDIX
-
REFERENCES
-
RELATED REPORTS
-
-
LIST OF TABLES
-
LIST OF ASSUMPTIONS & LIMITATIONS
-
PRIMARY INTERVIEWS AND INFORMATION GATHERING PROCESS
-
GLOBAL: Functional Service Providers (FSP) Market, BY TYPE, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: Functional Service Providers (FSP) Market, BY STAGE, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: Functional Service Providers (FSP) Market, BY APPLICATION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
GLOBAL: FUNCTIONAL SERVICE PROVIDERS MARKET, Functional Service Providers (FSP) Market, BY REGION, 2019-2032 (USD BILLION)
-
Functional Service Providers (FSP) Market, BY REGION, 2019–2032 (USD BILLION)
-
NORTH AMERICA: Functional Service Providers (FSP) Market, BY COUNTRY, 2019–2032 (USD BILLION)
-
NORTH AMERICA: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
NORTH AMERICA: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
NORTH AMERICA: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
US: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
US: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
US: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
CANADA: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
CANADA: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
CANADA: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
EUROPE: Functional Service Providers (FSP) Market, BY COUNTRY, 2019–2032 (USD BILLION)
-
EUROPE: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
EUROPE: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
EUROPE: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
GERMANY: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
GERMANY: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
GERMANY: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
FRANCE: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
FRANCE: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
FRANCE: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
UK: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
UK: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
UK: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
ITALY: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
ITALY: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
ITALY: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
SPAIN: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
SPAIN: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
SPAIN: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
REST OF EUROPE: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
REST OF EUROPE: Functional Service Providers (FSP) Market, BY STAGE, 2019–2032 (USD BILLION)
-
REST OF EUROPE: Functional Service Providers (FSP) Market, BY APPLICATION, 2019–2032 (USD BILLION)
-
ASIA-PACIFIC: Functional Service Providers (FSP) Market, BY COUNTRY, 2019–2032 (USD BILLION)
-
ASIA-PACIFIC: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
ASIA-PACIFIC: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
ASIA-PACIFIC: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
CHINA: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
CHINA: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
CHINA: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
JAPAN: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
JAPAN: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
JAPAN: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
INDIA: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
INDIA: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
INDIA: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
AUSTRALIA: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
AUSTRALIA: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
AUSTRALIA: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
SOUTH KOREA: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
SOUTH KOREA: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
SOUTH KOREA: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
REST OF ASIA-PACIFIC: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
REST OF ASIA-PACIFIC: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
REST OF ASIA-PACIFIC: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
REST OT THE WORLD: Functional Service Providers (FSP) Market, BY REGION, 2019–2032 (USD BILLION)
-
REST OF THE WORLD: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
REST OF THE WORLD: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
REST OF THE WORLD: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
LATIN AMERICA: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
LATIN AMERICA: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
LATIN AMERICA: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
MIDDLE EAST: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
MIDDLE EAST: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
MIDDLE EAST: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
AFRICA: Functional Service Providers (FSP) Market, BY TYPE, 2019–2032 (USD BILLION)
-
AFRICA: FUNCTIONAL SERVICE PROVIDERS MARKET BY STAGE, 2019–2032 (USD BILLION)
-
AFRICA: FUNCTIONAL SERVICE PROVIDERS MARKET BY APPLICATION, 2019–2032 (USD BILLION)
-
MAJOR PLAYERS IN THE GLOBAL FUNCTIONAL SERVICE PROVIDERS MARKET
-
MOST ACTIVE PLAYERS IN THE GLOBAL FUNCTIONAL SERVICE PROVIDERS MARKET
-
PRODUCT LAUNCHES
-
PARTNERSHIPS
-
ACQUISITIONS
-
IQVIA INC.: SOLUTIONS/SERVICES OFFERED
-
IQVIA INC.: KEY DEVELOPMENTS
-
LABORATORY CORPORATION OF AMERICA HOLDINGS: PRODUCTS/SERVICES OFFERED
-
LABORATORY CORPORATION OF AMERICA HOLDINGS: KEY DEVELOPMENTS
-
ICON PLC: PRODUCTS/SERVICES OFFERED
-
KPS LIFE, LLC: PRODUCTS/SERVICES OFFERED
-
WUXI APPTEC CO., LTD.: SOLUTIONS/SERVICES OFFERED
-
WUXI APPTEC CO., LTD.: KEY DEVELOPMENTS
-
PPD INC.: SOLUTIONS/SERVICES OFFERED
-
PAREXEL INTERNATIONAL CORPORATION: PRODUCTS/SERVICES OFFERED

















Leave a Comment