Friedreich's Ataxia Drug Market Overview
As per MRFR analysis, the Friedreich’s Ataxia Drug Market Size was estimated at 1.15 (USD Billion) in 2024. The Friedreich’s Ataxia Drug Market Industry is expected to grow from 1.29 (USD Billion) in 2025 to 3.47 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 11.62% during the forecast period (2025 - 2034).
Key Friedreich's Ataxia Drug Market Trends Highlighted
The Friedreich's Ataxia Drug Market is witnessing significant growth driven by a combination of factors. One major driver is the increasing prevalence of Friedreich's Ataxia, which has led to a higher demand for effective treatments. As awareness of the disease grows within the medical community and among patients, there is a greater emphasis on developing targeted therapies. Advances in biotechnology and pharmacology have also fostered innovation in drug development, enabling the creation of treatments that specifically address the underlying causes of the condition. Additionally, government initiatives and funding for rare diseases play a crucial role in supporting research and development efforts.There are considerable opportunities to be explored within the market, particularly in the area of personalized medicine. Tailoring treatments based on individual genetic profiles could enhance efficacy and safety, making a significant impact on patient outcomes. Collaborations between pharmaceutical companies, research institutions, and patient advocacy groups can further bridge gaps in knowledge and resources, creating a more robust support system for clinical trials and drug development. Emerging markets also present an untapped potential, as increased healthcare access can lead to greater awareness and diagnosis of Friedreich's Ataxia in previously underserved regions.Recent trends show a shift toward gene therapy and stem cell research as promising avenues for treatment. Innovative approaches are being explored, and clinical trials focusing on these methods are gaining momentum. The rise of digital health technologies is also influencing patient management and monitoring, providing additional support for those with Friedreich's Ataxia. Emphasizing the need for continued education and collaboration among stakeholders will be essential in fostering advancements and improving the quality of life for affected individuals. Overall, the market is at a pivotal point, characterized by evolving treatments and a dedicated focus on meeting the needs of patients.
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
Friedreich's Ataxia Drug Market Drivers
Increasing Prevalence of Friedreich's Ataxia
The Friedreich's Ataxia Drug Market Industry is witnessing growth driven by the increasing prevalence of Friedreich's Ataxia (FA) globally. This rare genetic disease affects the nervous system and the heart, leading to progressive loss of coordination, muscle weakness, and other debilitating symptoms. As the awareness of this condition grows among healthcare providers and the public, more patients are being diagnosed, which in turn fuels demand for effective treatment options.Furthermore, advancements in genetic testing and screening methods allow for early detection of FA, contributing to an increased number of patients seeking treatment. The growing patient population drives investment in research and development by pharmaceutical companies, leading to the introduction of innovative therapies specifically targeted at managing and treating symptoms of FA. The rise in clinical trials and the development of new drugs also indicate a bustling market environment, promoting growth.With a projected increase in the number of diagnosed cases, the market is expected to flourish, reinforcing the opportunity for drug manufacturers to explore and expand their portfolios aimed at addressing the needs of this patient demographic.
Technological Advancements in Drug Development
Technological advancements in drug development are significantly propelling the Friedreich's Ataxia Drug Market Industry forward. With the emergence of new biopharmaceutical technologies, there is a greater ability to develop more targeted therapies that align with the biological mechanisms of Friedreich's Ataxia. These innovations encompass gene therapy, small molecules, and the use of biomarkers to tailor treatments. As the understanding of FA at a molecular and genetic level deepens, researchers are better equipped to create effective therapies, driving market growth.Additionally, improved manufacturing processes and higher efficiency in clinical trials contribute to faster drug approvals and accessibility for patients.
Increased Funding for Rare Disease Research
The Friedreich's Ataxia Drug Market Industry benefits significantly from increased funding and investments directed towards rare disease research. Various stakeholders, including government bodies, private organizations, and non-profits, are recognizing the urgent need to develop effective treatments for rare diseases like Friedreich's Ataxia. Increased financial support has created a conducive environment for research, fostering collaborations between academic institutions and pharmaceutical companies.This influx of funding has enabled more extensive clinical trials and the exploration of novel therapeutic approaches, which ultimately promise to enhance treatment options available to patients, thus driving market growth.
Friedreich's Ataxia Drug Market Segment Insights:
Friedreich's Ataxia Drug Market Drug Type Insights
The Friedreich's Ataxia Drug Market revenue showcases a dynamic landscape segmented by Drug Type, where each segment plays a crucial role in the treatment and management of this rare disease. In 2023, the total market is valued at 0.93 USD Billion, reflecting the growing demand for effective therapies. Among the segments, Symptomatic Treatment accounted for 0.3 USD Billion in 2023, and its projected value is expected to rise to 0.8 USD Billion by 2032, highlighting its majority holding in providing immediate relief to patients. The importance of symptomatic treatments is evident as they address the various complications and symptoms associated with Friedreich's Ataxia, ensuring improved quality of life for those affected.Meanwhile, Disease-Modifying Treatment, valued at 0.4 USD Billion in 2023, is anticipated to reach 1.1 USD Billion in 2032, indicating a notable rise and a significant focus on research and development aimed at altering the disease's progression. This segment is crucial as it not only helps manage symptoms but also targets the underlying mechanisms of Friedreich's Ataxia, making it a vital aspect of comprehensive care. Nutritional Supplements, although smaller, showing a valuation of 0.23 USD Billion in 2023, are projected to grow to 0.6 USD Billion by 2032, which emphasizes the growing recognition of supportive therapies in nutritional management for patients.Supplements play a significant role in addressing specific deficiencies and supporting overall health, which is critical given the metabolic challenges faced by patients with Friedreich's Ataxia. The combined dynamics of these segments illustrate the evolving nature of treatments available and underscore the importance of continuous advancements and investments in the Friedreich's Ataxia Drug Market segmentation. Overall, the market growth is driven by rising awareness, ongoing research initiatives, and a pressing need for effective treatments, highlighting a positive outlook for these drug types in the coming years.The Friedreich's Ataxia Drug Market statistics indicate an expanding interest among pharmaceutical companies to invest in therapies that can provide hope and better outcomes for patients battling this debilitating condition.
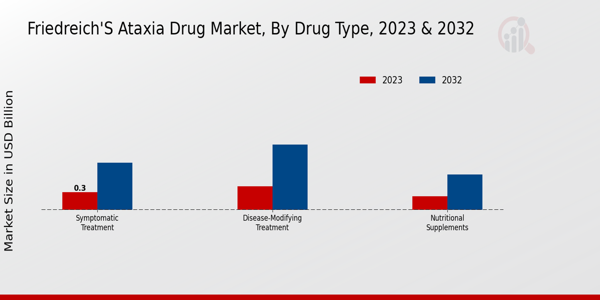
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
Friedreich's Ataxia Drug Market Administration Route Insights
The Friedreich's Ataxia Drug Market is projected to be valued at 0.93 USD Billion in 2023 and is set for significant growth in the coming years. The Administration Route segment plays a crucial role in the overall market dynamics, with various means of delivering medication leading to differing efficacy and patient adherence. Among these methods, the Oral route is noteworthy, as it is often preferred by patients due to ease of use and greater comfort. Meanwhile, the Intravenous route is significant for its rapid onset of action, making it vital for acute treatment settings.Subcutaneous administration is also gaining traction, primarily due to the convenience it offers for self-administration at home. As the market expands, the focus on optimizing these administration methods continues to grow, aligning with trends favoring patient-centric solutions. Overall, the segmentation of the Friedreich's Ataxia Drug Market revenue highlights the importance of tailored delivery systems to improve treatment outcomes, offering various avenues for driving market growth, navigating challenges, and seizing opportunities in the industry.The steady rise of each administration route emphasizes their unique benefits, contributing to an increasingly diverse treatment landscape.
Friedreich's Ataxia Drug Market Therapeutic Area Insights
The Friedreich's Ataxia Drug Market within the Therapeutic Area segment is poised for significant growth, characterized by a market value of 0.93 billion USD in 2023 and projected to reach 2.5 billion USD by 2032. In this market, the Neurology sector plays a crucial role, as Friedreich's Ataxia primarily affects the nervous system, leading to motor dysfunction and coordination issues. Additionally, Genetics is a vital area due to the hereditary nature of the disease, emphasizing the need for genetic research and targeted therapies. The Metabolism segment is also significant, focusing on the metabolic dysfunctions associated with Friedreich's Ataxia, opening pathways for novel drug development.Overall, Friedreich's Ataxia Drug Market revenue reflects the persistent efforts in research and innovation addressing complex biological mechanisms in these key therapeutic areas, while market growth is driven by increasing awareness and the resulting demand for effective treatment options. The insights derived from Friedreich's Ataxia Drug Market statistics demonstrate a well-segmented market landscape conducive to new opportunities and trends in developing therapeutics.
Friedreich's Ataxia Drug Market Patient Demographics Insights
The Friedreich's Ataxia Drug Market is experiencing significant growth driven by increasing awareness and advancements in treatment options, with a market value of 0.93 billion USD in 2023, expected to rise to 2.5 billion USD by 2032. The Patient Demographics segment showcases diverse age groups impacted by this condition, including Pediatric, Adult, and Geriatric populations, each representing unique challenges and requirements for treatment. The Adult demographic is particularly significant, as it typically encompasses individuals in their most productive years, highlighting the need for effective intervention strategies within this group.Pediatric patients are also crucial, as early diagnosis can greatly influence long-term outcomes and development. Meanwhile, the Geriatric population, though smaller, requires targeted therapies due to age-related complications. The Friedreich's Ataxia Drug Market revenue will likely benefit from these trends as ongoing research and clinical trials propel market growth forward. However, challenges such as the high costs of therapy and the need for specialized care may impact wider accessibility. Opportunities lie in the development of tailored treatments that address the specific needs of each demographic, enhancing the market landscape.The Friedreich's Ataxia Drug Market segmentation will continue to evolve as healthcare providers better understand the nuanced requirements of these diverse patient groups.
Friedreich's Ataxia Drug Market Regional Insights
The Friedreich's Ataxia Drug Market revenue is projected to grow significantly across various regions, with North America leading by a substantial margin. In 2023, the North American market was valued at 0.5 USD Billion, expected to rise to 1.25 USD Billion by 2032, showcasing its dominant position, which is attributed to advanced healthcare infrastructure and increased research activities. Europe follows, initially valued at 0.25 USD Billion and anticipated to reach 0.7 USD Billion in 2032, reflecting a strong interest in innovative treatment options amid a supportive regulatory environment.The Asia-Pacific (APAC) region, while currently smaller at 0.1 USD Billion, is on a growth trajectory, reaching 0.3 USD Billion by 2032, driven by rising healthcare expenditures and growing awareness. South America, valued at 0.05 USD Billion in 2023, is projected to experience modest growth to 0.15 USD Billion, highlighting emerging opportunities despite local healthcare challenges. The Middle East and Africa (MEA) is expected to grow from 0.03 USD Billion to 0.1 USD Billion, indicating the potential for market expansion as healthcare systems improve.The Friedreich's Ataxia Drug Market statistics emphasize that the North American and European markets hold the majority share, making them critical in shaping the future of treatments for Friedreich's Ataxia.
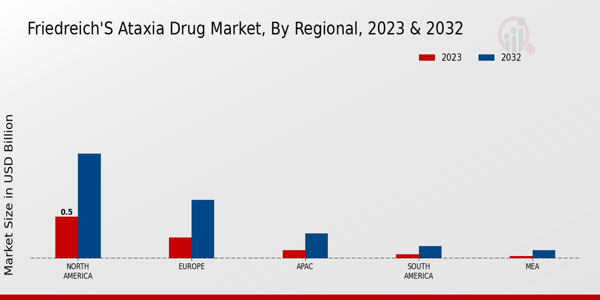
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
Friedreich's Ataxia Drug Market Key Players and Competitive Insights:
The Friedreich's Ataxia Drug Market is characterized by its niche focus within the wider landscape of neurological disorders, specifically targeting the complex nature of Friedreich's Ataxia. This progressive, inherited condition primarily affects the nervous system and the heart, necessitating specialized therapeutic solutions. The competitive dynamics in this market are influenced by a variety of factors, including the efficacy and safety profiles of treatments, ongoing clinical trials, regulatory approvals, and collaborative efforts among biopharmaceutical companies and research institutions. With an increasing prevalence of rare diseases drawing attention from healthcare providers, investors, and governments, the market is emerging as a critical area for innovation and strategic partnerships aimed at developing effective therapies.Ionis Pharmaceuticals holds a robust position within the Friedreich's Ataxia Drug Market, driven by its pioneering work in antisense technology. The company's focus on targeted RNA therapeutics differentiates it in a space where traditional treatment options remain limited. Ionis Pharmaceuticals is committed to enhancing therapeutic efficacy while minimizing adverse effects, addressing the unique needs of Friedreich's Ataxia patients. The company’s innovative pipeline includes promising candidates that are designed to modify disease progression rather than merely alleviate symptoms, which could significantly improve the quality of life for affected individuals. With a focus on strategic collaborations and scientific research, Ionis Pharmaceuticals continues to strengthen its market presence, leveraging its proprietary technologies to advance the development of effective drugs tailored to this rare neurological disorder.Pfizer is another key player in the Friedreich's Ataxia Drug Market, capitalizing on its extensive experience and resources in pharmaceutical research and development. The company has a diversified portfolio that enables it to engage in multiple therapeutic areas, including neurology. Pfizer’s commitment to developing groundbreaking therapies for rare diseases, including Friedreich's Ataxia, is evident through its investments in research and collaborations with academic institutions. The company leverages its capabilities in clinical trial design and regulatory expertise to advance its investigational therapies. By focusing on patient-centered approaches and expanding access to treatments, Pfizer aims to make a significant impact in the lives of individuals diagnosed with Friedreich's Ataxia, showcasing its dedication to addressing the unmet needs within this specialized market segment.
Key Companies in the Friedreich's Ataxia Drug Market Include:
-
Ionis Pharmaceuticals
-
Pfizer
-
Apexian Pharmaceuticals
-
Bayer
-
Biogen
-
Eisai
-
Cure Genetics
-
Amgen
-
Sarepta Therapeutics
-
Orphan Pharmaceuticals
-
Genentech
-
Acorda Therapeutics
-
Novartis
-
Sanofi
-
CB Therapeutics
Friedreich's Ataxia Drug Market Industry Developments
Recent developments in the Friedreich's Ataxia Drug Market have highlighted significant progress among leading companies. Ionis Pharmaceuticals has made strides in RNA-targeted therapies, enhancing treatment options for Friedreich's Ataxia. Pfizer continues to engage in research and collaborations aimed at addressing the needs of patients affected by this condition. Apexian Pharmaceuticals has gained attention for its innovative approaches to drug development in this area. Meanwhile, Bayer and Biogen are focusing on clinical trials to evaluate the efficacy of their respective therapies. In terms of mergers and acquisitions, there have been notable collaborations among these major players, aiming to expand their portfolios and reach in Friedreich's Ataxia market. Companies like Amgen and Sarepta Therapeutics are also exploring strategic partnerships to leverage their resources for drug advancement. The market is witnessing an upward trend in valuation, driven by the growing interest in targeted therapies, which is creating a competitive landscape that fosters innovation. This increased focus on research and development is expected to further shape Friedreich's Ataxia treatment landscape, impacting patient access and overall market dynamics.
Friedreich's Ataxia Drug Market Segmentation Insights
-
Friedreich's Ataxia Drug Market Drug Type Outlook
-
Friedreich's Ataxia Drug Market Administration Route Outlook
-
Oral
-
Intravenous
-
Subcutaneous
-
Friedreich's Ataxia Drug Market Therapeutic Area Outlook
-
Neurology
-
Genetics
-
Metabolism
-
Friedreich's Ataxia Drug Market Patient Demographics Outlook
-
Pediatric
-
Adult
-
Geriatric
-
Friedreich's Ataxia Drug Market Regional Outlook
-
North America
-
Europe
-
South America
-
Asia Pacific
-
Middle East and Africa
| Report Attribute/Metric |
Details |
|
Market Size 2024
|
1.15 (USD Billion)
|
|
Market Size 2025
|
1.29 (USD Billion)
|
|
Market Size 2034
|
3.47 (USD Billion)
|
|
Compound Annual Growth Rate (CAGR)
|
11.62 % (2025 - 2034)
|
|
Report Coverage
|
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends
|
|
Base Year
|
2024
|
|
Market Forecast Period
|
2025 - 2034
|
|
Historical Data
|
2020 - 2024
|
| Market Forecast Units |
USD Billion |
| Key Companies Profiled |
Ionis Pharmaceuticals, Pfizer, Apexian Pharmaceuticals, Bayer, Biogen, Eisai, Cure Genetics, Amgen, Sarepta Therapeutics, Orphan Pharmaceuticals, Genentech, Acorda Therapeutics, Novartis, Sanofi, CB Therapeutics |
| Segments Covered |
Drug Type, Administration Route, Therapeutic Area, Patient Demographics, Regional |
| Key Market Opportunities |
Innovative gene therapies development, Collaboration with research institutions, Expansion into emerging markets, Increased awareness and diagnosis, Support for patient advocacy groups |
| Key Market Dynamics |
Increasing patient population, Advancements in gene therapy, Rising investment in R, Growing awareness and diagnosis, Emerging collaboration among stakeholders |
| Countries Covered |
North America, Europe, APAC, South America, MEA |
Frequently Asked Questions (FAQ) :
The Friedreich's Ataxia Drug Market is expected to be valued at 3.47 billion USD in 2034.
The expected CAGR for the Friedreich's Ataxia Drug Market from 2025 to 2034 is 11.62 %.
North America is projected to hold the largest market share, valued at 1.25 billion USD in 2032.
The market size for Symptomatic Treatment is expected to reach 0.8 billion USD by 2032.
Key players include Ionis Pharmaceuticals, Pfizer, Biogen, Bayer, and Sarepta Therapeutics.
The expected market size for Disease-Modifying Treatment is projected to be 1.1 billion USD by 2032.
The APAC region is anticipated to reach a market value of 0.3 billion USD by 2032.
Nutritional Supplements are expected to have a market value of 0.6 billion USD by 2032.
Growth opportunities stem from increasing awareness, innovation in drug development, and unmet medical needs.
The market size for the South America region is projected to reach 0.15 billion USD by 2032.
















