Market Share
Esophageal Squamous Cell Carcinoma Market Share Analysis
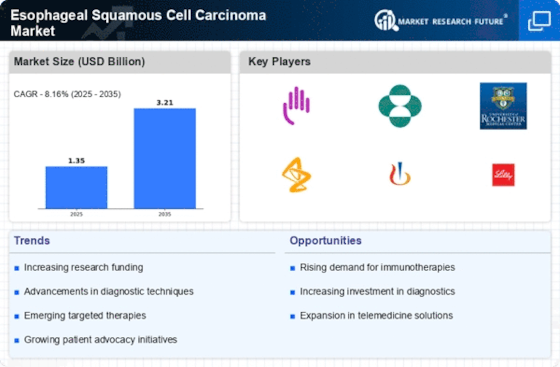
Advances in cancer research, diagnosis and treatments for esophageal squamous cell carcinoma (ESCC) have brought about significant changes in ESCC market. The aggressive nature of this subtype of esophageal cancer presents challenges as it has few treatment options. There is a growing focus on personalized care and targeted therapy for patients with ESCC, which is evident from molecular profiling being done by scientists to identify various mutations and alterations in the tumor and thus pave way for personalized therapies that are able to specifically address the unique characteristic of a particular patient’s cancer.
Similarly, the development of immunotherapies is rapidly increasing in the ESCC Market. As per clinical trials, immune checkpoint inhibitors such as pembrolizumab and nivolumab have demonstrated favorable outcomes for ESCC. These drugs are meant to recruit body’s immunity against cancer cells which according to studies represents a major breakthrough within the treatment scenario of esophageal squamous cell carcinoma. Immunotherapy however seems to offer some hope as possible durable responders may be realized that shall lead to improved overall survival rates among patients whose disease is advanced.
Furthermore, there have been advancements regarding early detection methods and diagnostic strategies within the market. Like other cancers, early stage diagnosis is crucial in improving outcomes of ESCC. For instance there are ongoing efforts aimed at improving endoscopic techniques, molecular imaging systems and liquid biopsy approaches so as to increase sensitivity and specificity respectively during early detection times. These discoveries contribute towards formulation of better screening programs as well as diagnostic tools for cases concerning esophageal squamous cell carcinoma.
ESCC Market has been significantly influenced through collaborations between pharmaceutical companies; research institutions alongside healthcare organizations These partnerships create room for collaborative research projects involving joint clinical trials while new therapeutic interventions designed purposely for treating individuals with squamous cell carcinoma can also be developed at this point out collaboration occurs through partnerships. Besides more investments into translational research and biomarker discovery have led to the continuous development of precision medicine within ESCC.
The market is now focusing on combined therapies as there is an expansion in knowledge about molecular and genetic mechanisms underlying ESCC. Therefore, efforts are being aimed at combining targeted agents, immunotherapies as well as traditional chemotherapy regimens used in the treatment of esophageal cancer with the aim of making them more effective and successful in overcoming resistance problems. Consequently, rational combination strategies can improve overall therapeutic outcomes for patients suffering from such disease.
Esophageal cancer is a rising global health burden particularly in regions like Asia and Africa that have high rates of incidence. Steps are also being taken to address disparities in diagnosis, treatment access and care delivery. The market has explored better avenues through which patients can be engaged while digital health solutions including telemedicine have been proposed to help facilitate remote consultations while increasing accessibility to latest advances made in ESCC management.

















Leave a Comment