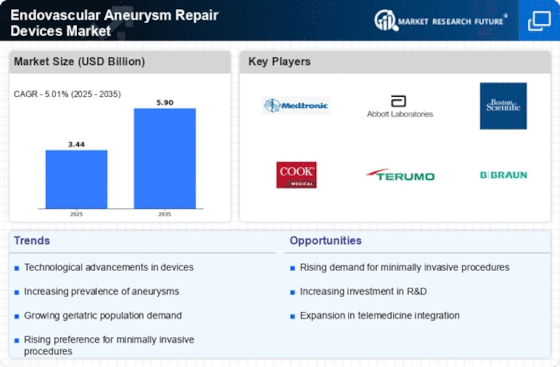
Top Industry Leaders in the Endovascular Aneurysm Repair Devices Market

Latest Endovascular aneurysm repair devices Companies Updates
Jan 2024
Medtronic Received FDA approval for their Endurant II AAA Stent-graft System, featuring enhanced deliverability and conformability for complex aortic anatomies.Partnering with hospitals and research institutions on developing next-generation EVAR solutions with improved long-term outcomes.
Cook Medical Launched their Zenith Flex AAA Stent-graft System, offering a flexible and conformable design for challenging anatomy cases.Collaborating with healthcare providers on clinical trials and educational programs to promote broader adoption of EVAR for diverse patient populations.
Terumo Introduced their Jotec Aortic Branch Stent-graft System for repairing aneurysms involving aortic branches, addressing a less common but challenging anatomical variation.Focusing on expanding their EVAR portfolio with solutions for complex AAA cases and improving procedural efficiency.
W.L. Gore & Associates Received CE mark approval for their EXCLUDER ViP AAA System, showcasing continued innovation in stent-graft designs with enhanced features for secure anchoring and long-term durability.Investing in research and development of novel materials and technologies for EVAR devices to optimize clinical outcomes.
Other Players Companies like Getinge Group, CryoLife (Jotec GmbH), and BiFlow Medical are also contributing to market growth with diverse EVAR device offerings and targeted solutions for specific needs.
List of Endovascular aneurysm repair devices Key companies in the market
- Novartis Pharmaceuticals,
- Hoffmann-La Roche,
- Principia Biopharma Inc,
- Argenx, Sanofi S.A.,
- Pfizer Inc.,
- Teligent Inc,
- Glaxo Smith Kline LLC,
- Teva Pharmaceutical Industries