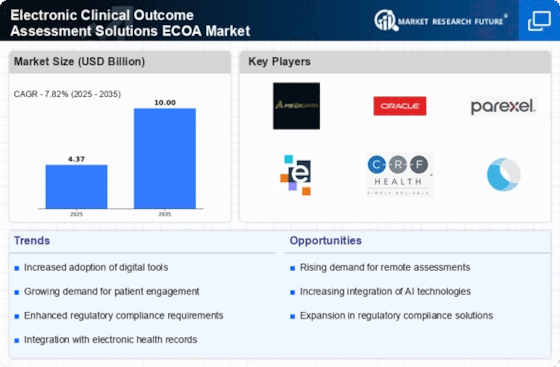
-
EXECUTIVE SUMMARY
-
Market Overview
-
Key Findings
-
Market Segmentation
-
Competitive Landscape
-
Challenges and Opportunities
-
Future Outlook
-
MARKET INTRODUCTION
-
Definition
-
Scope of the study
- Research Objective
- Assumption
- Limitations
-
RESEARCH METHODOLOGY
-
Overview
-
Data Mining
-
Secondary Research
-
Primary Research
-
Primary Interviews and Information Gathering Process
-
Breakdown of Primary
-
Respondents
-
Forecasting Model
-
Market Size Estimation
-
Bottom-Up Approach
-
Top-Down Approach
-
Data Triangulation
-
Validation
-
MARKET DYNAMICS
-
Overview
-
Drivers
-
Restraints
-
Opportunities
-
MARKET FACTOR ANALYSIS
-
Value chain Analysis
-
Porter's Five Forces
-
Analysis
-
Bargaining Power of Suppliers
- Bargaining Power
-
of Buyers
-
Threat of New Entrants
- Threat of Substitutes
- Intensity of Rivalry
-
COVID-19 Impact Analysis
-
Market Impact Analysis
-
Regional Impact
- Opportunity and
-
Threat Analysis
-
Electronic Clinical Outcome Assessment
-
Solutions ECOA Market, BY Deployment Mode (USD Billion)
-
Cloud-Based
-
On-Premise
-
Hybrid
-
Electronic Clinical Outcome Assessment
-
Solutions ECOA Market, BY Type of Assessment (USD Billion)
-
Patient-Reported
-
Outcomes
-
Clinician-Reported Outcomes
-
Observer-Reported Outcomes
-
Performance Outcomes
-
Electronic Clinical Outcome Assessment
-
Solutions ECOA Market, BY End User (USD Billion)
-
Pharmaceutical Companies
-
Contract Research Organizations
-
Medical Device Manufacturers
-
Healthcare Providers
-
Electronic Clinical Outcome Assessment
-
Solutions ECOA Market, BY Application (USD Billion)
-
Clinical Trials
-
Post-Market Surveillance
-
Real-World Evidence Generation
-
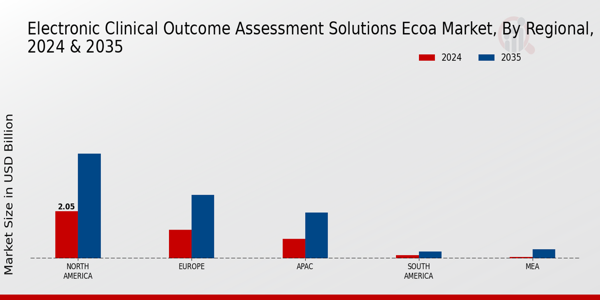
Electronic Clinical Outcome Assessment Solutions ECOA Market, BY Regional (USD Billion)
-
North America
- US
- Canada
-
Europe
- Germany
- UK
- France
- Russia
- Italy
- Spain
- Rest of Europe
-
APAC
-
China
- India
- Japan
- South
-
Korea
-
Malaysia
- Thailand
- Indonesia
- Rest of APAC
-
South America
- Brazil
-
Mexico
-
Argentina
- Rest of South America
-
MEA
- GCC Countries
- South Africa
- Rest of MEA
-
Competitive Landscape
-
Overview
-
Competitive Analysis
-
Market share Analysis
-
Major Growth
-
Strategy in the Electronic Clinical Outcome Assessment Solutions ECOA Market
-
Competitive Benchmarking
-
Leading Players in Terms of Number
-
of Developments in the Electronic Clinical Outcome Assessment Solutions ECOA Market
-
Key developments and growth strategies
- New Product Launch/Service
-
Deployment
-
Merger & Acquisitions
- Joint Ventures
-
Major Players Financial Matrix
- Sales and Operating Income
- Major Players R&D Expenditure. 2023
-
Company Profiles
-
Clinical Ink
- Financial Overview
- Products
-
Offered
-
Key Developments
- SWOT Analysis
-
Key Strategies
-
Castor EDC
- Financial Overview
-
Products Offered
-
Key Developments
- SWOT Analysis
- Key Strategies
-
WCG Clinical
- Financial Overview
- Products Offered
- Key Developments
- SWOT
-
Analysis
-
Key Strategies
-
Medpace
- Financial
-
Overview
-
Products Offered
- Key Developments
-
SWOT Analysis
-
Key Strategies
-
eClinical Solutions
-
Financial Overview
-
Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
PAREXEL International
- Financial Overview
- Products Offered
- Key
-
Developments
-
SWOT Analysis
- Key Strategies
-
CRF Health
-
Financial Overview
- Products Offered
-
Key Developments
-
SWOT Analysis
- Key Strategies
-
Alira Health
-
Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key Strategies
-
Comprehend Systems
- Financial Overview
- Products
-
Offered
-
Key Developments
- SWOT Analysis
-
Key Strategies
-
OPTUM
- Financial Overview
-
Products Offered
-
Key Developments
- SWOT Analysis
- Key Strategies
-
ERT
- Financial Overview
- Products Offered
- Key Developments
- SWOT
-
Analysis
-
Key Strategies
-
IQVIA
- Financial
-
Overview
-
Products Offered
- Key Developments
-
SWOT Analysis
-
Key Strategies
-
Medidata Solutions
- Financial Overview
- Products Offered
- Key
-
Developments
-
SWOT Analysis
- Key Strategies
-
Celerion
-
Financial Overview
- Products Offered
-
Key Developments
-
SWOT Analysis
- Key Strategies
-
Veristat
- Financial Overview
- Products Offered
- Key Developments
- SWOT Analysis
- Key
-
Strategies
-
Appendix
-
References
-
Related Reports
-
America Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
America Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
America Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT
-
MODE, 2019-2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035
-
(USD Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
US Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE
-
OF ASSESSMENT, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY END USER,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Europe Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY END USER, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
UK Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY REGIONAL, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT
-
MODE, 2019-2035 (USD Billions)
-
Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Russia Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY APPLICATION, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035
-
(USD Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Italy Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE
-
OF ASSESSMENT, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY END USER,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD
-
Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD
-
Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
APAC Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY REGIONAL, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT
-
MODE, 2019-2035 (USD Billions)
-
Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
India Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY APPLICATION, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035
-
(USD Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Japan Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
Korea Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY REGIONAL, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT
-
MODE, 2019-2035 (USD Billions)
-
Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Thailand Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY APPLICATION, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035
-
(USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035
-
(USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Rest of APAC Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE
-
ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Rest of APAC Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
America Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY END USER, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Mexico Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY END USER, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION,
-
2035 (USD Billions)
-
Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE,
-
2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE
-
OF ASSESSMENT, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY END USER, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY APPLICATION, 2019-2035 (USD Billions)
-
Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES &
-
FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST,
-
BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE
-
OF ASSESSMENT, 2019-2035 (USD Billions)
-
Outcome Assessment Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY END USER,
-
2035 (USD Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD
-
Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD
-
Billions)
-
Solutions ECOA Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
ECOA Market SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
Market SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Market SIZE ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY DEPLOYMENT MODE, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY TYPE OF ASSESSMENT, 2019-2035 (USD Billions)
-
SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
Rest of MEA Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE
-
ESTIMATES & FORECAST, BY APPLICATION, 2019-2035 (USD Billions)
-
Rest of MEA Electronic Clinical Outcome Assessment Solutions ECOA Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
DEVELOPMENT/APPROVAL
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
BY TYPE OF ASSESSMENT
-
SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
US ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
ANALYSIS BY DEPLOYMENT MODE
-
SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
CANADA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY APPLICATION
-
ECOA MARKET ANALYSIS BY REGIONAL
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
GERMANY ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY TYPE OF ASSESSMENT
-
SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
GERMANY ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY
-
REGIONAL
-
MARKET ANALYSIS BY DEPLOYMENT MODE
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
UK ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END
-
USER
-
ANALYSIS BY APPLICATION
-
SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
FRANCE ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY TYPE OF ASSESSMENT
-
SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
FRANCE ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY
-
REGIONAL
-
ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
RUSSIA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY END USER
-
ECOA MARKET ANALYSIS BY APPLICATION
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT
-
MODE
-
MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
ANALYSIS BY REGIONAL
-
SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
ANALYSIS BY END USER
-
SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
REST OF EUROPE ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET
-
ANALYSIS BY DEPLOYMENT MODE
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
REST OF EUROPE ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET
-
ANALYSIS BY END USER
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
EUROPE ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY
-
REGIONAL
-
ECOA MARKET ANALYSIS
-
SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
ANALYSIS BY END USER
-
SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
INDIA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY DEPLOYMENT MODE
-
SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
INDIA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY APPLICATION
-
ECOA MARKET ANALYSIS BY REGIONAL
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF
-
ASSESSMENT
-
ECOA MARKET ANALYSIS BY END USER
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
MARKET ANALYSIS BY DEPLOYMENT MODE
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
SOUTH KOREA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY END USER
-
SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
MALAYSIA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY DEPLOYMENT MODE
-
SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
MALAYSIA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY APPLICATION
-
SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
ANALYSIS BY TYPE OF ASSESSMENT
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
MARKET ANALYSIS BY REGIONAL
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF
-
ASSESSMENT
-
ECOA MARKET ANALYSIS BY END USER
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
INDONESIA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY REGIONAL
-
SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF
-
ASSESSMENT
-
SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
REST OF APAC ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY REGIONAL
-
SOLUTIONS ECOA MARKET ANALYSIS
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF
-
ASSESSMENT
-
ECOA MARKET ANALYSIS BY END USER
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
ANALYSIS BY DEPLOYMENT MODE
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
MEXICO ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY
-
END USER
-
ECOA MARKET ANALYSIS BY APPLICATION
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
ARGENTINA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY DEPLOYMENT MODE
-
SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
MARKET ANALYSIS BY APPLICATION
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
SOUTH AMERICA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY DEPLOYMENT MODE
-
ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
REST OF SOUTH AMERICA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET
-
ANALYSIS BY END USER
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY APPLICATION
-
REST OF SOUTH AMERICA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET
-
ANALYSIS BY REGIONAL
-
SOLUTIONS ECOA MARKET ANALYSIS
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
GCC COUNTRIES ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET
-
ANALYSIS BY TYPE OF ASSESSMENT
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
GCC COUNTRIES ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY APPLICATION
-
SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
SOUTH AFRICA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY APPLICATION
-
SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY DEPLOYMENT MODE
-
MARKET ANALYSIS BY TYPE OF ASSESSMENT
-
OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS BY END USER
-
REST OF MEA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET ANALYSIS
-
BY APPLICATION
-
SOLUTIONS ECOA MARKET ANALYSIS BY REGIONAL
-
OF ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET
-
RESEARCH PROCESS OF MRFR
-
ASSESSMENT SOLUTIONS ECOA MARKET
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET
-
ANALYSIS: ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET
-
SUPPLY / VALUE CHAIN: ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA
-
MARKET
-
BY DEPLOYMENT MODE, 2024 (% SHARE)
-
ASSESSMENT SOLUTIONS ECOA MARKET, BY DEPLOYMENT MODE, 2019 TO 2035 (USD Billions)
-
BY TYPE OF ASSESSMENT, 2024 (% SHARE)
-
ASSESSMENT SOLUTIONS ECOA MARKET, BY TYPE OF ASSESSMENT, 2019 TO 2035 (USD Billions)
-
BY END USER, 2024 (% SHARE)
-
SOLUTIONS ECOA MARKET, BY END USER, 2019 TO 2035 (USD Billions)
-
ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET, BY APPLICATION, 2024
-
(% SHARE)
-
MARKET, BY APPLICATION, 2019 TO 2035 (USD Billions)
-
CLINICAL OUTCOME ASSESSMENT SOLUTIONS ECOA MARKET, BY REGIONAL, 2024 (% SHARE)
-
TO 2035 (USD Billions)