Dystrophic Epidermolysis Bullosa Treatment Market Overview
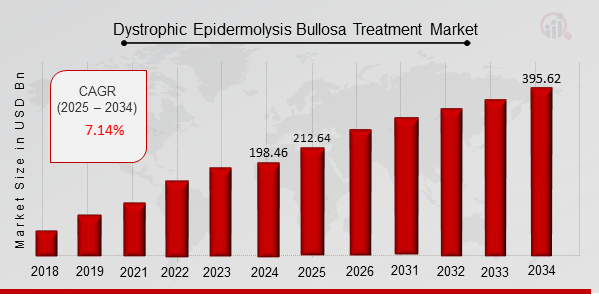
As per MRFR analysis, the Dystrophic Epidermolysis Bullosa Treatment Market Size was estimated at 198.46 (USD Billion) in 2024. The Dystrophic Epidermolysis Bullosa Treatment Market Industry is expected to grow from 212.64 (USD Billion) in 2025 to 395.62 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 7.14% during the forecast period (2025 - 2034).
Key Dystrophic Epidermolysis Bullosa Treatment Market Trends Highlighted
The increasing prevalence of dystrophic epidermolysis bullosa drives the growth of the treatment market. Technological advancements in gene therapy, such as gene editing and RNA interference, present opportunities for effective treatments.
The rising adoption of precision medicine approaches enables personalized treatment plans based on genetic profiles. Emerging strategies like tissue engineering and cell therapy hold promise for regenerative therapies.
Recent trends include collaborations between academia and industry to accelerate research and development, as well as government initiatives to support innovation in this field.
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
Dystrophic Epidermolysis Bullosa Treatment Market Drivers
Rising Prevalence of Dystrophic Epidermolysis Bullosa
Dystrophic epidermolysis bullosa is an uncommon genetic skin disease that makes the skin of a person fragile and vulnerable to blistering. In the world, the incidence of DEB ranges up to approximately 1 in 50,000 people. Nowadays, one of the most important factors that is spurring the market growth of the Dystrophic Epidermolysis Bullosa Treatment Market is the increasing incidence of DEB.
There are a number of factors that can be attributed to the growing incidence of the disease, such as increased recognition of the disease, advancement of diagnostic techniques, and faster development of new technologies in the medical sphere, among others.
This growth of the disease is beneficial for the market expansion because it indicates that the market demand for the treatment procedures will rise as well. In addition to the increasing incidence of skin disease, a number of brand-new drug products that are on the market today positively influence market growth.
There have been a number of breakthroughs in the treatment DEB area in recent years, and it has resulted in the introduction of actual cures for the disease, such as gene therapy and stem cell therapy. It is essential to note that these two technologies, among others, have a positive influence on the rise of the population living with the DEB treatment market in the forthcoming period.
Growing Demand for Specialized Wound Care Products
People who develop DEB need specialized wound care products to treat and prevent their blisters and wounds. There is a wide range of wound care products that include different types of dressings, bandages, and other medical supplies that are used to protect the skin and help it heal.
The increasing demand for these specialized wound care products is a key driver promoting the growth in the Global Dystrophic Epidermolysis Bullosa Treatment Market. The demand for these products is expected to keep on growing in the future, because the prevalence of DEB is increasing and new innovative products are being introduced.
Furthermore, the development of the market in the developing countries due to wider availability of specialized wound care products is expected to be a driving factor.
Government Funding and Support for DEB Research
Government funding and support for DEB research is another major factor driving the growth of the Global Dystrophic Epidermolysis Bullosa Treatment Market. Governments around the world are increasingly recognizing the importance of DEB research, and they are providing funding for clinical trials and other research projects.
This funding is helping to drive the development of new and innovative treatments for DEB, and it is expected to continue to drive the growth of the market in the coming years.
Dystrophic Epidermolysis Bullosa Treatment Market Segment Insights:
Dystrophic Epidermolysis Bullosa Treatment Market Treatment Type Insights
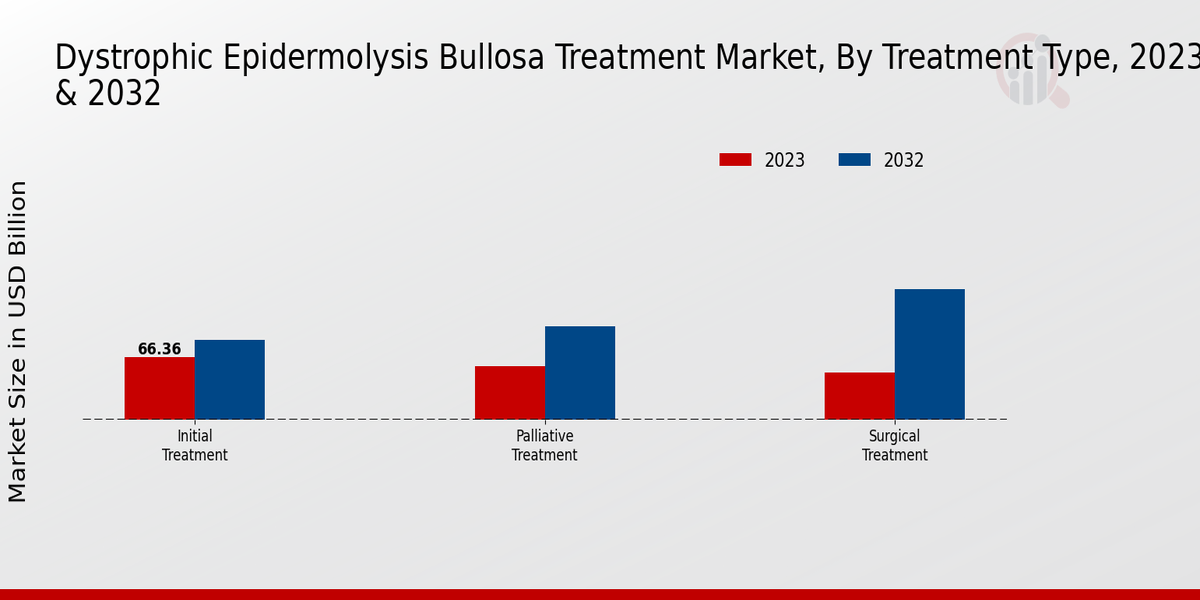
The Dystrophic Epidermolysis Bullosa Treatment Market had a varied treatment type segmentation comprising Initial Treatment, Palliative Treatment, and Surgical Treatment.
Within this market landscape, Initial Treatment emerged as a significant component, valued at 70.0 USD Billion in 2023 and expected to reach 130.0 USD Billion by 2032, indicating its majority holding role in the overall market dynamics.
This signified the critical early interventions that were essential for managing the symptoms and complications associated with this rare condition, highlighting the emphasis placed on timely and effective approaches in patient care.
Palliative Treatment, capturing a market value of 50.0 USD Billion in 2023 and projected to enhance to 95.0 USD Billion by 2032, played a crucial part in improving the quality of life for patients with Dystrophic Epidermolysis Bullosa. Its importance lies in providing symptomatic relief and psychological support, which can have a profound impact on the well-being of affected individuals and their families.
Surgical Treatment, valued at 52.88 USD Billion in 2023 and anticipated to grow to 96.5 USD Billion by 2032, held considerable weight in the treatment matrix, especially for individuals requiring corrective procedures to address complications or deformities resulting from the disease.
The increasing demand for surgical interventions illustrates the growing awareness and advancements in medical technologies that facilitate better surgical outcomes for patients. The overall segment insights reflect a market growth driven by a combination of increasing patient population, advancements in treatment methodologies, and the rising acceptance of specialized care strategies.
Each treatment type plays a significant role in addressing the unique needs of Dystrophic Epidermolysis Bullosa patients, showcasing a comprehensive approach to managing this condition while driving the evolution of the Dystrophic Epidermolysis Bullosa Treatment Market Statistics.
As the market continues to progress, opportunities for innovative therapies and integrated treatment plans are expected to emerge, thereby enhancing the overall patient experience in the future.
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
Dystrophic Epidermolysis Bullosa Treatment Market Route of Administration Insights
The segment plays a crucial role in how therapies are delivered to patients, impacting both efficacy and patient compliance.
Among the various routes, the oral administration method has gained popularity for its convenience and ease of use, significantly appealing to patients and caregivers. Topical treatments remain a key area, as they directly address skin lesions, offering localized relief with minimal systemic effects.
Additionally, injectables are noteworthy due to their targeted approach in delivering medications, often resulting in quick onset of action, making them popular in acute management scenarios.
The diversity in administration routes is a significant driver for market growth, catering to different patient needs and preferences. As the market continues to expand, understanding these dynamics within the Dystrophic Epidermolysis Bullosa Treatment Market segmentation will be essential for stakeholders aiming to improve therapeutic outcomes.
Dystrophic Epidermolysis Bullosa Treatment Market End User Insights
The End User aspect of this market includes critical sectors such as Hospitals, Clinics, and care settings, each playing a vital role in providing care for patients with dystrophic epidermolysis bullosa.
Hospitals often dominate this segment due to their capability to provide comprehensive treatment options, including surgeries and specialized care. Clinics serve as an essential middle ground for outpatient management, offering convenient options for regular check-ups and minor procedures.
Homecare Settings are gaining traction as they provide personalized care tailored to the patient's comfort, enabling better management of the condition in a familiar environment. The trends in these settings focus on enhancing accessibility and ensuring patient-centric approaches, which remain crucial for the Dystrophic Epidermolysis Bullosa Treatment Market revenue.
As the market evolves, there are abundant opportunities for innovative treatments and care delivery models that cater to the varying needs of patients across these End User platforms.
Dystrophic Epidermolysis Bullosa Treatment Market Dystrophic Epidermolysis Bullosa Subtype Insights
The Dystrophic Epidermolysis Bullosa Treatment Market showcases notable growth potential within its Dystrophic Epidermolysis Bullosa Subtype segment. This segment includes various forms, primarily EBS Simplex, EBS Dystrophica, and EBS Junctional, each holding significant influence in terms of prevalence and treatment needs.
The EBS Dystrophica subtype often requires specialized therapies due to its severe manifestations, making it a crucial focus for market development. Meanwhile, EBS Simplex presents a more common form but still demands effective treatment solutions, contributing to the steadily expanding market.
The insights derived from Dystrophic Epidermolysis Bullosa Treatment Market data illustrate a complex landscape where increasing awareness and advancements in therapeutic technologies drive the market forward.
The anticipated growth trajectory emphasizes the need for innovative treatment options, while the challenges related to patient diagnosis and access to healthcare services remain pertinent.
Overall, the health industry's response to these conditions is likely to shape the evolving dynamics of the Global Dystrophic Epidermolysis Bullosa Treatment Market, aligning with growing patient safety and efficacy standards.
Dystrophic Epidermolysis Bullosa Treatment Market Regional Insights
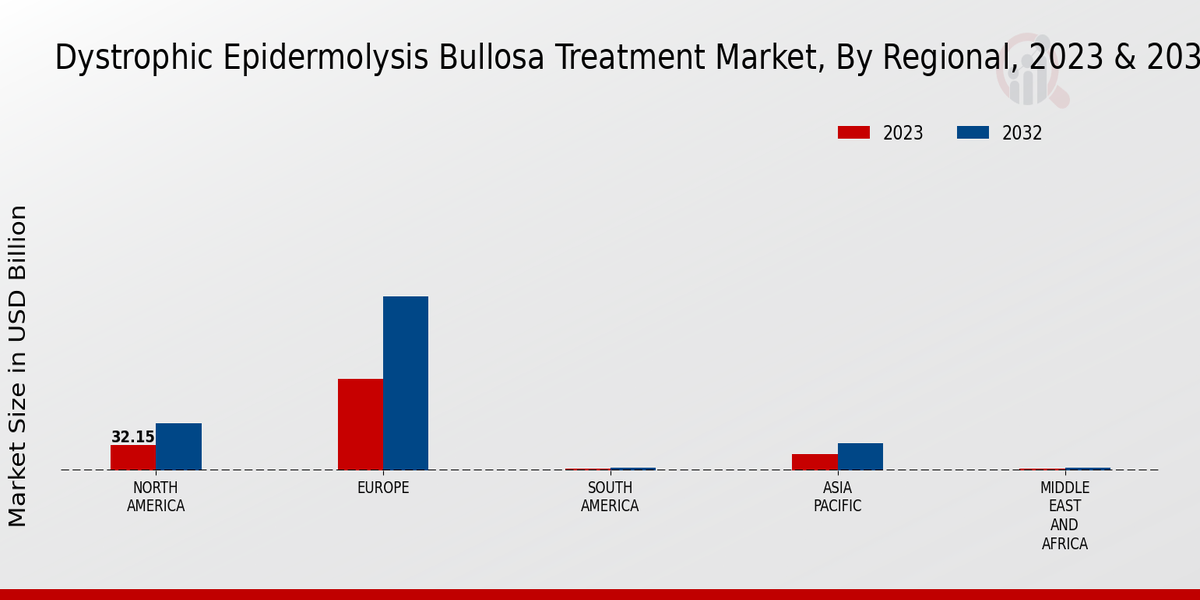
The Dystrophic Epidermolysis Bullosa Treatment Market exhibits a robust market presence across various regions, demonstrating diverse valuations. In 2023, North America led with a significant market revenue of 70.0 USD Billion, which is expected to double by 2032, highlighting its majority holding in the overall market.
Europe followed with a valuation of 50.0 USD Billion in 2023, reflecting considerable market activity and treatment development initiatives. The Asia Pacific region represented a growing segment valued at 30.0 USD Billion, showcasing increasing awareness and healthcare advancements, which are crucial for market growth.
South America, with a market revenue of 15.0 USD Billion, exhibited potential for expansion due to rising healthcare accessibility. The Middle East and Africa, although currently valued at 7.88 USD Billion, showed opportunities for growth as healthcare systems develop further.
These regional insights indicate varying levels of investment and focus on Dystrophic Epidermolysis Bullosa Treatment, driven by factors such as population health needs, healthcare infrastructure, and research funding, contributing to a dynamic and evolving landscape in the Global Dystrophic Epidermolysis Bullosa Treatment Market.
Source: Primary Research, Secondary Research, MRFR Database and Analyst Review
Dystrophic Epidermolysis Bullosa Treatment Market Key Players And Competitive Insights:
Major players in the Dystrophic Epidermolysis Bullosa Treatment Market are continuously developing new and innovative treatments to meet the needs of patients. These companies are also investing in clinical trials to evaluate the safety and efficacy of their products.
The leading Dystrophic Epidermolysis Bullosa Treatment Market players are focused on developing treatments that can improve the quality of life for patients and their families. The Dystrophic Epidermolysis Bullosa Treatment Market is expected to continue to grow in the coming years, driven by the increasing prevalence of the condition and the development of new and effective treatments.
Verrica Pharmaceuticals is a leading company in the Dystrophic Epidermolysis Bullosa Treatment Market. The company's lead product, VP-001, is a topical ointment that is used to treat the symptoms of dystrophic epidermolysis bullosa. VP-001 is a safe and effective treatment that can help to improve the quality of life for patients.
A competitor to Verrica Pharmaceuticals is Abeona Therapeutics. Abeona Therapeutics is a clinical-stage biopharmaceutical company that is developing gene therapies for rare diseases. The company's lead product, EB-101, is a gene therapy that is being developed to treat dystrophic epidermolysis bullosa. EB-101 is a promising treatment that could potentially provide a cure for dystrophic epidermolysis bullosa.
Key Companies in the Dystrophic Epidermolysis Bullosa Treatment Market Include:
- Amryt Pharma
- Abeona Therapeutics
- Spark Therapeutics
- Vuna Pharmaceuticals
- Canterbury Medical
- Mesoblast
- Prometic Life Sciences
- Kalgene Pharmaceuticals
- Mustang Bio
- Novartis Gene Therapies
Dystrophic Epidermolysis Bullosa Treatment Market Developments
The increasing prevalence of dystrophic epidermolysis bullosa, growing awareness of advanced treatment options, and supportive government initiatives are driving the market growth. Key players are focusing on developing innovative therapies, such as gene therapy and stem cell therapy, to cater to the unmet medical needs of patients.
Recent news developments include the approval of Amryt Pharma's Mytesi (otelacicleb) in the US and the initiation of clinical trials for Abeona Therapeutics' EB-101 gene therapy. These advancements are expected to fuel market growth in the coming years.
Dystrophic Epidermolysis Bullosa Treatment Market Segmentation Insights
- Dystrophic Epidermolysis Bullosa Treatment Market Treatment Type Outlook
- Initial Treatment
- Palliative Treatment
- Surgical Treatment
- Dystrophic Epidermolysis Bullosa Treatment Market Route of Administration Outlook
- Dystrophic Epidermolysis Bullosa Treatment Market End User Outlook
- Hospitals
- Clinics
- Homecare Settings
- Dystrophic Epidermolysis Bullosa Treatment Market Dystrophic Epidermolysis Bullosa Subtype Outlook
- EBS Simplex
- EBS Dystrophica
- EBS Junctional
- Dystrophic Epidermolysis Bullosa Treatment Market Regional Outlook
- North America
- Europe
- South America
- Asia Pacific
- Middle East and Africa
|
Report Attribute/Metric
|
Details
|
|
Market Size 2024
|
198.46 (USD Billion)
|
|
Market Size 2025
|
212.64 (USD Billion)
|
|
Market Size 2034
|
395.62 (USD Billion)
|
|
Compound Annual Growth Rate (CAGR)
|
7.14 % (2025 - 2034)
|
|
Report Coverage
|
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends
|
|
Base Year
|
2024
|
|
Market Forecast Period
|
2025 - 2034
|
|
Historical Data
|
2020 - 2024
|
|
Market Forecast Units
|
USD Billion
|
|
Key Companies Profiled
|
Amryt Pharma, Abeona Therapeutics, Spark Therapeutics, Vuna Pharmaceuticals, Canterbury Medical, Mesoblast, Prometic Life Sciences, Kalgene Pharmaceuticals, Mustang Bio, Novartis Gene Therapies
|
|
Segments Covered
|
Treatment Type, Route of Administration, End User, Dystrophic Epidermolysis Bullosa Subtype, Regional
|
|
Key Market Opportunities
|
Development of gene therapies Advancement in stem cell therapies Rising prevalence of DEB
|
|
Key Market Dynamics
|
Increasing prevalence Gene therapy advancements Rising RampD investments Emerging markets growth Growing awareness
|
|
Countries Covered
|
North America, Europe, APAC, South America, MEA
|
Frequently Asked Questions (FAQ) :
The Dystrophic Epidermolysis Bullosa Treatment Market is expected to be valued at 321.5 USD Billion in 2032.
The expected CAGR for the Dystrophic Epidermolysis Bullosa Treatment Market is 7.14% from 2025 to 2034.
North America is anticipated to have the largest market share, valued at 140.0 USD Billion in 2032.
The market size for the initial treatment of Dystrophic Epidermolysis Bullosa is expected to reach 130.0 USD Billion in 2032.
Key players in the market include Amryt Pharma, Abeona Therapeutics, Spark Therapeutics, and Novartis Gene Therapies.
The expected market value for palliative treatment is 95.0 USD Billion in 2032.
The anticipated market size for surgical treatment is expected to be 96.5 USD Billion in 2032.
The South American market is projected to grow to 30.0 USD Billion by 2032, up from 15.0 USD Billion in 2023.
The Asia Pacific region is expected to be valued at 60.0 USD Billion in 2032.
Challenges in the market include high treatment costs and the complexity of managing the condition.

















