Advancements in Gene Therapy
The Duchenne Muscular Dystrophy Treatment Market is witnessing a notable surge in advancements related to gene therapy. This innovative approach aims to address the underlying genetic causes of Duchenne Muscular Dystrophy, potentially altering the disease's trajectory. Recent studies indicate that gene therapy could significantly improve muscle function and prolong mobility in affected individuals.
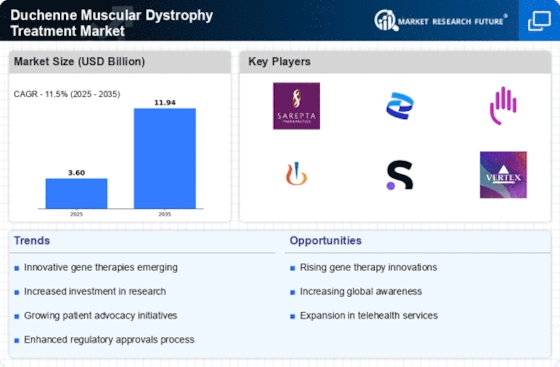
As of 2025, the market for gene therapy in this sector is projected to reach substantial figures, driven by increasing investments in research and development. Pharmaceutical companies are actively pursuing clinical trials, with several candidates showing promising results. This trend not only enhances treatment options but also raises awareness about Duchenne Muscular Dystrophy, thereby expanding the patient population that may benefit from these therapies.
Increased Awareness and Advocacy
The Duchenne Muscular Dystrophy Treatment Market is benefiting from increased awareness and advocacy efforts. Organizations dedicated to raising awareness about Duchenne Muscular Dystrophy are playing a crucial role in educating the public and healthcare professionals about the disease. This heightened awareness is leading to earlier diagnosis and intervention, which are critical for improving patient outcomes. As advocacy groups collaborate with researchers and pharmaceutical companies, the market is likely to see a rise in funding for research initiatives. By 2025, the impact of these efforts is expected to manifest in a broader range of treatment options available to patients, thereby enhancing the overall landscape of the Duchenne Muscular Dystrophy Treatment Market.
Growing Demand for Personalized Medicine
The Duchenne Muscular Dystrophy Treatment Market is increasingly influenced by the growing demand for personalized medicine. Tailoring treatments to individual patient profiles is becoming a focal point in healthcare, particularly for complex conditions like Duchenne Muscular Dystrophy. This approach allows for more effective management of the disease, as therapies can be customized based on genetic, environmental, and lifestyle factors. The market is expected to see a rise in the development of targeted therapies that align with this trend. As of 2025, the personalized medicine segment within the Duchenne Muscular Dystrophy Treatment Market is anticipated to grow significantly, reflecting a shift towards more individualized care strategies that enhance patient outcomes.
Collaborative Research and Development Efforts
The Duchenne Muscular Dystrophy Treatment Market is significantly influenced by collaborative research and development efforts among various stakeholders. Partnerships between academic institutions, pharmaceutical companies, and non-profit organizations are fostering an environment conducive to innovation. These collaborations aim to accelerate the discovery and development of new therapies, addressing the urgent need for effective treatments for Duchenne Muscular Dystrophy. As of 2025, the market is expected to benefit from an increase in joint ventures and funding initiatives that support research projects. This collaborative approach not only enhances the scientific understanding of the disease but also streamlines the pathway for bringing new treatments to market, ultimately benefiting patients and healthcare providers alike.
Technological Innovations in Treatment Delivery
The Duchenne Muscular Dystrophy Treatment Market is experiencing a wave of technological innovations that enhance treatment delivery methods. Advances in drug delivery systems, such as nanotechnology and sustained-release formulations, are improving the efficacy of existing therapies. These innovations facilitate targeted delivery of medications directly to affected muscle tissues, potentially increasing treatment effectiveness while minimizing side effects. As of 2025, the integration of technology in treatment protocols is likely to reshape the market landscape, making therapies more accessible and effective for patients. This trend underscores the importance of continuous research and development in the Duchenne Muscular Dystrophy Treatment Market, as stakeholders seek to leverage technology to improve patient care.