Increasing Regulatory Pressures
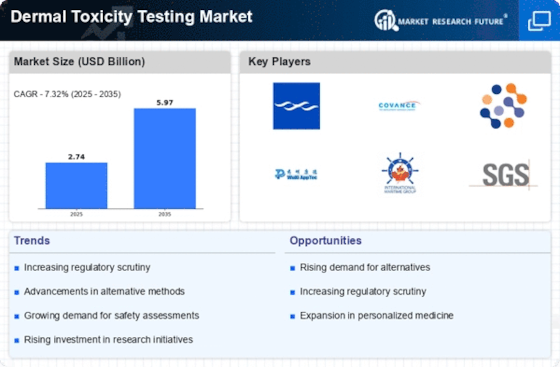
The Dermal Toxicity Testing Market is experiencing heightened regulatory pressures as governments and international organizations implement stricter guidelines for chemical safety. Regulatory frameworks such as REACH in Europe and the FDA regulations in the United States necessitate comprehensive dermal toxicity testing for a wide range of products. This trend is compelling manufacturers to adopt more rigorous testing protocols to ensure compliance and avoid potential legal repercussions. The market is projected to witness a surge in demand for dermal toxicity testing services, with an anticipated growth rate of 6% annually over the next five years. This increasing regulatory scrutiny is reshaping the landscape of dermal toxicity testing, driving innovation and investment in testing methodologies.
Rising Demand for Safety Assessments
The increasing emphasis on safety assessments in the Dermal Toxicity Testing Market is driven by heightened consumer awareness regarding product safety. As consumers become more informed about the potential risks associated with chemical exposure, companies are compelled to invest in comprehensive dermal toxicity testing. This trend is further supported by the growing number of regulatory bodies mandating rigorous safety evaluations for cosmetics, pharmaceuticals, and personal care products. In fact, the dermal toxicity testing market is projected to reach USD 1.5 billion by 2026, reflecting a compound annual growth rate of approximately 7.5%. This rising demand for safety assessments underscores the critical role of dermal toxicity testing in ensuring consumer protection and product efficacy.
Advancements in Vitro Testing Technologies
Technological innovations in vitro testing methods are significantly influencing the Dermal Toxicity Testing Market. The development of advanced cell cultures, organ-on-a-chip technologies, and high-throughput screening techniques has enhanced the accuracy and efficiency of dermal toxicity assessments. These innovations not only reduce the reliance on animal testing but also provide more relevant data regarding human skin responses. As a result, the market for in vitro testing is expected to grow substantially, with estimates suggesting a market size of USD 800 million by 2025. The integration of these advanced technologies into the dermal toxicity testing framework is likely to streamline the testing process and improve regulatory compliance.
Growing Pharmaceutical and Cosmetic Industries
The expansion of the pharmaceutical and cosmetic industries is a key driver of the Dermal Toxicity Testing Market. As these sectors continue to grow, the need for effective dermal toxicity testing becomes increasingly critical to ensure product safety and efficacy. The pharmaceutical industry, in particular, is projected to reach USD 1.3 trillion by 2025, necessitating robust testing protocols to comply with regulatory standards. Similarly, the cosmetic industry is expected to grow at a CAGR of 5.3%, further fueling the demand for dermal toxicity testing services. This growth trajectory indicates a sustained need for reliable testing solutions, positioning the dermal toxicity testing market as an essential component of product development and safety assurance.
Consumer Preference for Natural and Organic Products
The rising consumer preference for natural and organic products is significantly impacting the Dermal Toxicity Testing Market. As consumers increasingly seek products free from synthetic chemicals, manufacturers are compelled to ensure that their formulations are safe and non-toxic. This shift towards natural ingredients necessitates thorough dermal toxicity testing to validate the safety of these products. The market for organic personal care products is projected to reach USD 25 billion by 2025, indicating a substantial opportunity for dermal toxicity testing services. This trend not only reflects changing consumer attitudes but also highlights the importance of rigorous testing in maintaining product integrity and consumer trust.