Chemotherapeutic
Non-Chemotherapeutics
Gravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Sterile
Non-Sterile
With Robotic Arms
Without Robotic Arms
North America Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
North America Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
North America Compounding Chemotherapy by SterilitySterile
Non-Sterile
North America Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
US Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
US Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
US Compounding Chemotherapy by SterilitySterile
Non-Sterile
US Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
CANADA Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
CANADA Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
CANADA Compounding Chemotherapy by SterilitySterile
Non-Sterile
CANADA Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Europe Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Europe Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Europe Compounding Chemotherapy by SterilitySterile
Non-Sterile
Europe Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Germany Outlook (USD Billion, 2018-2030)
Germany Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Germany Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Germany Compounding Chemotherapy by SterilitySterile
Non-Sterile
Germany Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
France Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
France Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
France Compounding Chemotherapy by SterilitySterile
Non-Sterile
France Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
UK Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
UK Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
UK Compounding Chemotherapy by SterilitySterile
Non-Sterile
UK Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
ITALY Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
ITALY Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
ITALY Compounding Chemotherapy by SterilitySterile
Non-Sterile
ITALY Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Spain Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Spain Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Spain Compounding Chemotherapy by SterilitySterile
Non-Sterile
Spain Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Rest Of Europe Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
REST OF EUROPE Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
REST OF EUROPE Compounding Chemotherapy by SterilitySterile
Non-Sterile
REST OF EUROPE Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Asia-Pacific Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Asia-Pacific Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Asia-Pacific Compounding Chemotherapy by SterilitySterile
Non-Sterile
Asia-Pacific Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
China Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
China Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
China Compounding Chemotherapy by SterilitySterile
Non-Sterile
China Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Japan Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Japan Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Japan Compounding Chemotherapy by SterilitySterile
Non-Sterile
Japan Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
India Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
India Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
India Compounding Chemotherapy by SterilitySterile
Non-Sterile
India Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Australia Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Australia Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Australia Compounding Chemotherapy by SterilitySterile
Non-Sterile
Australia Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Rest of Asia-Pacific Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Rest of Asia-Pacific Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Rest of Asia-Pacific Compounding Chemotherapy by SterilitySterile
Non-Sterile
Rest of Asia-Pacific Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Rest of the World Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Rest of the World Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Rest of the World Compounding Chemotherapy by SterilitySterile
Non-Sterile
Rest of the World Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Middle East Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Middle East Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Middle East Compounding Chemotherapy by SterilitySterile
Non-Sterile
Middle East Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Africa Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Africa Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Africa Compounding Chemotherapy by SterilitySterile
Non-Sterile
Africa Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
Latin America Compounding Chemotherapy by DoseChemotherapeutic
Non-Chemotherapeutics
Latin America Compounding Chemotherapy by Delivery MethodGravimetric Automated Compounding Device
Volumetric Automated Compounding Device
Latin America Compounding Chemotherapy by SterilitySterile
Non-Sterile
Latin America Compounding Chemotherapy by TechnologyWith Robotic Arms
Without Robotic Arms
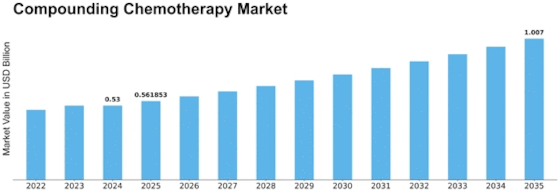


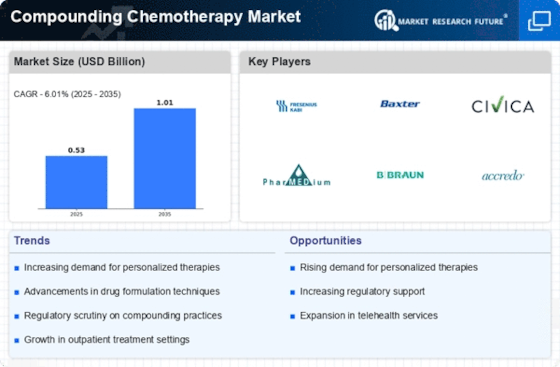















Leave a Comment