Market Trends
Key Emerging Trends in the Clinical Genomics Market
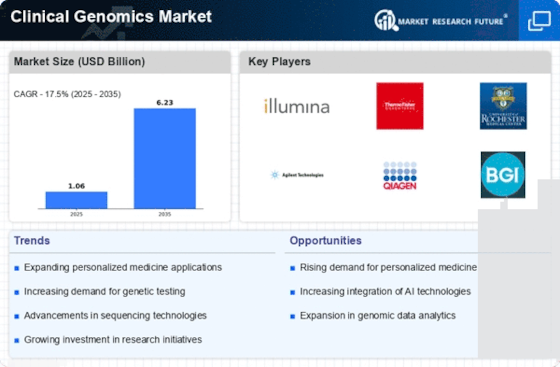
The Clinical Genomics market is witnessing a surge in demand due to the increasing awareness of customized remedies. As genomic information will become extra on hand and less costly, healthcare companies are leveraging genetic facts to tailor treatment plans based on the characteristics of the affected person. Rapid trends in sequencing technologies are riding market developments. Next-generation sequencing (NGS) systems have come to be more green and cost-effective, making an allowance for large-scale genomic analysis. This has led to accelerated adoption in scientific settings, enabling extra complete genetic checking out. Liquid biopsies, a non-invasive approach to obtaining genetic facts from bodily fluids, are gaining traction in Clinical Genomics. These assessments are especially valuable in cancer diagnostics, allowing early detection and monitoring of genetic mutations without the need for invasive methods. Clinical genomics is being carried out more and more to improve the prognosis and remedy uncommon diseases. Genetic testing enables the discovery of uncommon genetic variations liable for these conditions, facilitating early intervention and customized treatment plans for sufferers with restrained healing alternatives. Pharmacogenomics, the take a look at ways a man or woman's genetic makeup impacts their response to pills, is gaining prominence. Tailoring drug prescriptions based totally on genetic factors improves treatment efficacy and minimizes unfavorable reactions, using the adoption of pharmacogenomic checking out. Collaborations between pharmaceutical agencies, studies institutions, and diagnostic laboratories are on the rise. These partnerships purpose to accelerate the improvement of novel healing procedures, diagnostics, and genetic assessments, fostering innovation and expanding the Clinical Genomics market. Efforts to make genetic testing more available to a broader populace are influencing market dynamics. Reduced prices, coupled with consciousness campaigns, are encouraging individuals to go through genetic checking out for disease threat assessment, ancestry analysis, and for the purpose of making family plans. Regulatory frameworks are evolving to preserve tempo with the speedy improvements in Clinical Genomics. Governments and regulatory bodies are operating to establish clear guidelines for genetic checking out, ensuring the safety, accuracy, and ethical use of genomic information in healthcare settings. Direct-to-consumer (DTC) genetic checking out is gaining recognition as individuals are seeking to discover their genetic makeup independently. Companies offering DTC genetic trying offerings offer insights into ancestry, fitness predispositions, and provider fame, contributing to the growth of the patron genomics market.

















Leave a Comment