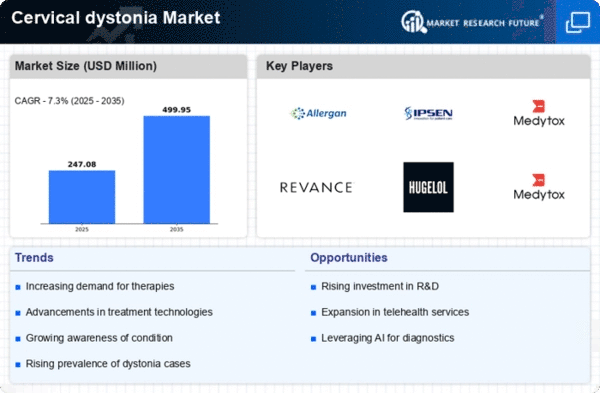
Top Industry Leaders in the Cervical dystonia Market

Revance Therapeutics' Daxxify Approved for Cervical Dystonia In a major win for the company, Revance Therapeutics received FDA approval for its long-acting botulinum toxin A treatment, Daxxify, for cervical dystonia in December 2023. This marks the first new toxin approved for the condition in over a decade and is expected to challenge AbbVie's market dominance with Botox.
Xeomin Granted Breakthrough Therapy Designation for Cervical Dystonia has been granted Breakthrough Therapy Designation by the FDA for the treatment of cervical dystonia. This designation speeds up the development and review process for promising new drugs that may address unmet medical needs.
Ipsen Advances Phase 3 Trial for ONT-385 for Cervical Dystonia announced positive interim results from its Phase 3 GALILEO study evaluating ONT-385, a novel, orally administered treatment for cervical dystonia. The drug showed statistically significant improvements in patients' head posture and disability scores compared to placebo.
Acorda Therapeutics Files BLA for INBRIJA for Cervical Dystonia submitted a Biologics License Application (BLA) to the FDA for INBRIJA (intrathecal baclofen), a non-surgical, sustained-release formulation of baclofen, for the treatment of cervical dystonia. The company expects a decision from the FDA in the second half of 2024.
Cynapsis Announces Positive Topline Results for Study of CINQA in Cervical Dystonia reported positive topline results from its Phase 2b CINQA-CD study evaluating its investigational drug, CINQA, for the treatment of cervical dystonia. The study met its primary and secondary endpoints, demonstrating CINQA's potential to improve patients' symptoms and quality of life.
List of Cervical dystonia Key Companies in the Market
- ALLERGAN (Ireland)
- Solstice Neurosciences LLC
- Ipsen Biopharmaceuticals Inc. (France)
- Merz Inc (US)
- Addex Therapeutics (Switzerland)
- Revance Therapeutics Inc. (US)