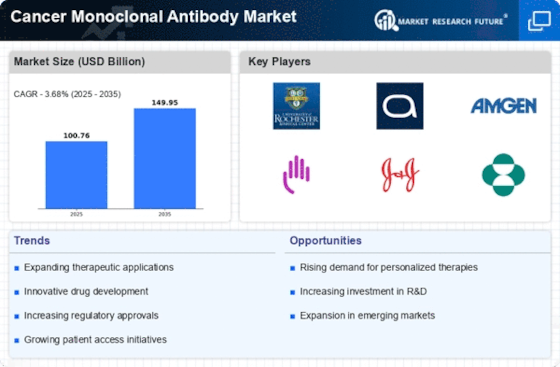
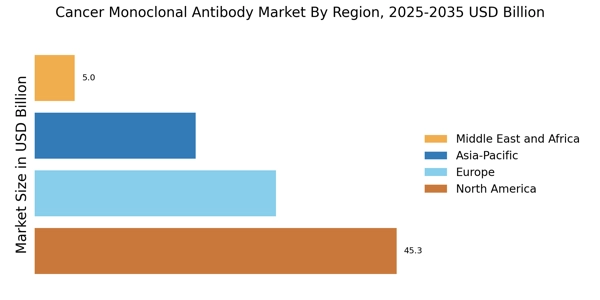
Growing Incidence of Cancer
The rising incidence of cancer worldwide is a primary driver for the Cancer Monoclonal Antibody Market. According to recent statistics, cancer cases are projected to increase significantly, with estimates suggesting that by 2040, the number of new cancer cases could reach 29.5 million. This alarming trend necessitates the development of innovative therapies, including monoclonal antibodies, which are increasingly recognized for their efficacy in targeting specific cancer cells. As healthcare systems strive to address this growing burden, investments in research and development of monoclonal antibodies are likely to surge, thereby propelling the market forward. Furthermore, the increasing awareness of cancer screening and early detection is expected to contribute to the demand for monoclonal antibody therapies, as patients seek more effective treatment options.
Advancements in Biotechnology
Technological advancements in biotechnology are significantly influencing the Cancer Monoclonal Antibody Market. Innovations in genetic engineering and recombinant DNA technology have enabled the development of highly specific monoclonal antibodies that can effectively target cancer cells while minimizing damage to healthy tissues. The introduction of bispecific antibodies and antibody-drug conjugates represents a new frontier in cancer treatment, enhancing the therapeutic potential of monoclonal antibodies. Moreover, the integration of artificial intelligence in drug discovery processes is streamlining the identification of promising candidates, potentially reducing the time and cost associated with bringing new therapies to market. As these technologies continue to evolve, they are expected to drive the growth of the Cancer Monoclonal Antibody Market, offering new hope for patients and healthcare providers alike.
Rising Demand for Targeted Therapies
The shift towards targeted therapies is a significant driver of the Cancer Monoclonal Antibody Market. Patients and healthcare providers are increasingly favoring treatments that offer specificity and reduced side effects compared to traditional chemotherapy. Monoclonal antibodies, known for their ability to target specific antigens on cancer cells, are gaining traction as effective alternatives. Market data indicates that the targeted therapy segment is expected to grow at a compound annual growth rate of over 10% in the coming years. This trend is further supported by the increasing number of clinical trials focusing on monoclonal antibodies, which are demonstrating promising results in various cancer types. As the demand for personalized and effective treatment options rises, the Cancer Monoclonal Antibody Market is poised for substantial growth.
Increasing Investment in Cancer Research
The Cancer Monoclonal Antibody Market is experiencing a surge in investment, driven by both public and private sectors. Governments and pharmaceutical companies are allocating substantial resources to cancer research, recognizing the urgent need for effective treatments. In 2023, global spending on cancer research reached approximately 200 billion USD, with a significant portion directed towards the development of monoclonal antibodies. This influx of funding is facilitating clinical trials and accelerating the approval process for new therapies. Additionally, partnerships between academic institutions and biotech firms are fostering innovation, leading to the discovery of novel monoclonal antibodies. As investment continues to grow, it is likely to enhance the competitive landscape of the Cancer Monoclonal Antibody Market, ultimately benefiting patients through improved treatment options.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly supportive of innovative therapies, which is positively impacting the Cancer Monoclonal Antibody Market. Initiatives aimed at expediting the approval process for breakthrough therapies are becoming more common, allowing monoclonal antibodies to reach the market faster. For instance, the FDA's Breakthrough Therapy Designation program has been instrumental in facilitating the development of promising cancer treatments. This regulatory environment encourages pharmaceutical companies to invest in monoclonal antibody research, as the path to market is becoming less cumbersome. Furthermore, the establishment of clear guidelines for the development and approval of biosimilars is expected to enhance competition within the Cancer Monoclonal Antibody Market, potentially leading to lower costs and increased accessibility for patients.