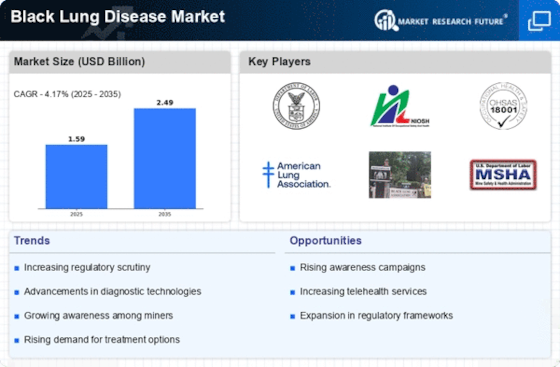
Top Industry Leaders in the Black Lung Disease Market
 Latest Black Lung Disease Companies Update
Latest Black Lung Disease Companies Update
November 2023: In India, Lupin Limited, a pharmaceutical giant, introduced Vilfuro-G, the first fixed-dose triple combination medication (FDC) in the world, for the treatment of chronic obstructive pulmonary disease (COPD). This significant step comes after the dry powder inhaler (DPI) product received clearance from the Drug Controller General of India. If you suffer from moderate to severe chronic obstructive pulmonary disease (COPD), you need a long-term solution. Lupin Vilfuro-G is the only FDC that combines all three medications. The medicine will be offered in a fixed-dose single-strength form, and the suggested dosage is once day. Beyond 37 million people in India are battling chronic obstructive pulmonary disease. It is one of the top causes of disability and mortality in the country.
July 2023: Boehringer Ingelheim has announced that their supplemental New Drug Application (sNDA) for OFEV (nintedanib) approved by the U.S. Food and Drug Administration (FDA). OFEV is being studied as a possible treatment for fibrosing interstitial lung disease (ILD) in children and adolescents aged 6 to 17. Childhood interstitial lung disorders can be a heavy burden on patients and their families due to the lengthy and complex diagnostic process and the lack of authorized treatments. If given the green light, OFEV would revolutionize the treatment landscape for fibrosing interstitial lung disease in children and teenagers (those between the ages of 6 and 17). The sNDA is derived on the findings of the InPedILD phase III study, which administered OFEV to children and adolescents (6–17 years old) with clinically significant fibrosing ILD. The trial assessed the safety, dose-exposure, and efficacy of OFEV in addition to standard of treatment.List of Black Lung Disease Key companies in the market
- Boehringer Ingelheim International GmbH (Germany)
- GlaxoSmithKline plc (U.K)
- Novartis AG (Switzerland)
- Teva Pharmaceutical Industries Ltd. (Israel)
- AstraZeneca (U.K)
- Koninklijke Philips N.V. (The Netherlands)
- GENERAL ELECTRIC (U.S.)
- Siemens AG (Germany)
- TOSHIBA CORPORATION (Japan)
- Medtronic (U.S.)