Top Industry Leaders in the Biomaterial Wound Dressing Market

Convatec (UK) Launched ConvaMax+ in July 2023, a new biomaterial dressing designed for highly exuding wounds and offering extended wear time. This addition strengthens their portfolio for managing challenging wounds.
Mölnlycke (Sweden) Received 510(k) clearance from the FDA for Mepilex® Transfer in August 2023, a silicone dressing with a unique transfer layer for minimizing pain and disruption during dressing changes. This technology improves patient experience and potentially accelerates healing.
Acelity (US) Expanded its KerraMax® Hydrofiber dressing line with the launch of KerraMax® Care in September 2023, offering a cost-effective option for managing moderate to highly exuding wounds. This caters to varied needs and budgets.
Urgo Group (France) Announced positive Phase 2a trial results for their UrgoStart® PBM (photobiomodulation) therapy in June 2023, demonstrating its potential to accelerate wound healing in diabetic foot ulcers. This innovative technology holds promise for a non-invasive treatment approach
Medtronic (US) Entered into a strategic partnership with Humacyte (US) in October 2023 to develop and commercialize Humacyte's acellular human tissue-based biomaterial platform for various medical applications, including wound healing. This collaboration combines expertise for potentially transformative solutions.
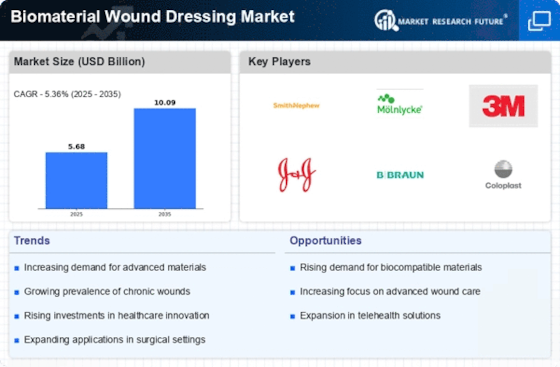
List of Biomaterial Wound Dressing Key Companies in the Market
- Smith & Nephew (UK)
- Braun Melsungen AG (Germany)
- 3M (US)
- Mölnlycke Health Care (Sweden)
- Acelity (US)
- ConvaTec, Inc (UK)
- Coloplast Corp (Denmark)
- Derma Sciences, Inc (US)
- Cardinal Health (US)
- Medtronic (Ireland)