Biologics Safety Testing Size
Biologics Safety Testing Market Growth Projections and Opportunities
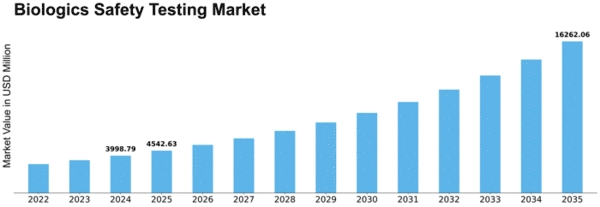
The Biologics Safety Testing Market length is expected to attain USD 9.76 Billion by means of 2032 at 13.6% CAGR throughout the forecast length of 2023-2032. The Biologics Safety Testing Market is formed with the aid of a multitude of factors that collectively outline its panorama and contribute to its growth. One of the number one drivers is the growing demand for biologics, including monoclonal antibodies, vaccines, and gene treatment options, in the pharmaceutical and biotechnology industries. As biologics grow to be integral to the treatment of numerous sicknesses, making sure their protection and efficacy through rigorous trying out is paramount. Technological advancements play a pivotal role in shaping the biologics safety testing market. Continuous innovations in analytical techniques, including next-generation sequencing, mass spectrometry, and excessive-throughput screening, contribute to the improvement of greater touchy and dependable strategies for Biologics Safety Testing. Stringent regulatory requirements extensively impact the Biologics Safety Testing Market. Regulatory groups, which include the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), establish recommendations and standards for the protection testing of biologics. The increasing complexity of biological production techniques, which include the usage of novel expression structures and mobile strains, contributes to the demand for superior protection trying out strategies. Biologics Safety Testing must adapt to the evolving panorama of bioprocessing, addressing the unique, demanding situations associated with diverse production platforms, inclusive of the mammalian mobile way of life, microbial fermentation, and cell-loose structures. The potential to increase bendy and platform-agnostic testing strategies is critical for meeting the numerous needs of biological producers. Economic considerations, such as price-effectiveness and the capability impact on manufacturing timelines, affect the adoption of Biologics Safety Testing strategies. While making sure the safety of biologic merchandise is non-negotiable, there's a regular power to optimize testing tactics, reduce charges, and expedite time-to-market. Global collaboration and harmonization of trying out requirements make contributions to advancements within the Biologics Safety Testing Market. Collaboration among regulatory bodies, enterprise stakeholders, and research establishments fosters the improvement of standardized trying-out strategies and pointers. Market opposition and consolidation play a sizable role in defining the dynamics of the biologics safety testing market. The presence of set up trying out carrier vendors, contract research organizations (CROs), and in-residence checking out centers make contributions to market opposition.


















Leave a Comment