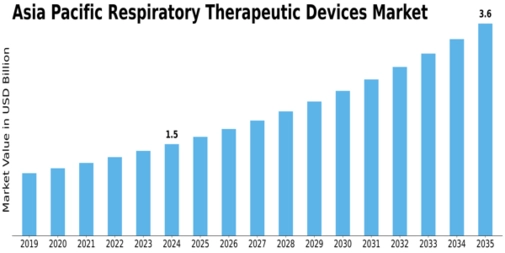
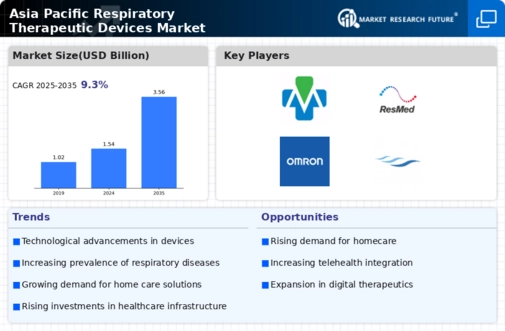
Asia Pacific Respirtory Therapeutic Devices Size
Asia Pacific Respirtory Therapeutic Devices Market Growth Projections and Opportunities
The Asia-Pacific respiratory therapeutic devices marketplace is witnessing a massive surge because of the growing occurrence of respiratory issues, including chronic obstructive pulmonary disease (COPD), asthma, and sleep apnea. The growing aging population, on the side of environmental elements, contributes to the escalating demand for respiratory therapeutic devices within the location. Ongoing technological advancements in respiratory therapeutic devices are propelling marketplace enlargement. Innovations, together with portable and person-friendly gadgets, clever inhalers, and telehealth solutions, are gaining traction, presenting patients with extra handy and powerful treatment alternatives. Growing consciousness about respiratory health and the significance of early prognosis and treatment is using marketplace increase. Educational campaigns and initiatives by means of healthcare agencies and governments are encouraging individuals to seek medical help for respiratory problems, contributing to the growing demand for healing gadgets. As disposable incomes increase throughout the Asia-Pacific place, people are more inclined to invest in their health. This has caused a surge in healthcare spending, such as the purchase of respiratory therapeutic devices, both by individuals and healthcare institutions. High rates of smoking and deteriorating air pollution contribute to the escalating occurrence of respiration disorders. This grim truth is fueling the demand for respiratory therapeutic devices as people are searching for powerful answers to manipulate and alleviate respiration signs due to environmental elements. Market gamers are accomplishing strategic collaborations and partnerships to bolster their market presence. This consists of collaborations with healthcare carriers, studies establishments, and other stakeholders to decorate product development, distribution networks, and average market attain. The regulatory surroundings perform a crucial role in shaping the Asia-Pacific respiratory therapeutic devices marketplace. Adherence to stringent regulatory requirements and compliance with great guarantee measures are vital for marketplace players to benefit and maintain consumer beliefs. A developing choice for domestic-primarily based healthcare answers is fostering the adoption of respiratory therapeutic devices. The Asia-Pacific vicinity is attracting global marketplace gamers seeking to capitalize on the increasing healthcare quarter. Increased market entry via global organizations brings in superior technologies and a broader range of respiratory therapeutic devices, intensifying opposition and using innovation within the market.






Leave a Comment