-
FACTOR ANALYSIS
-
\r\n\r\n\r\nValue chain Analysis
-
\r\n\r\n\r\nPorter's
-
Five Forces Analysis
-
\r\n\r\n\r\nBargaining Power of Suppliers
-
\r\n\r\n\r\nBargaining
-
Power of Buyers
-
\r\n\r\n\r\nThreat of New Entrants
-
\r\n\r\n\r\nThreat
-
of Substitutes
-
\r\n\r\n\r\nIntensity of Rivalry
-
\r\n\r\n\r\n\r\n\r\nCOVID-19
-
Impact Analysis
-
\r\n\r\n\r\nMarket Impact Analysis
-
\r\n\r\n\r\nRegional
-
Impact
-
\r\n\r\n\r\nOpportunity and Threat Analysis
-
\r\n\r\n\r\n\r\n\r\n\r\n\r\n
-
\r\n
-
\r\n\r\n\r\nAntiviral
-
Drugs Market, BY Drug Type (USD Billion)
-
\r\n\r\n\r\nNucleoside
-
Analogues
-
\r\n\r\n\r\nNon-Nucleoside Analogues
-
\r\n\r\n\r\nProtease
-
Inhibitors
-
\r\n\r\n\r\nNeuraminidase Inhibitors
-
\r\n\r\n\r\n\r\n\r\nAntiviral
-
Drugs Market, BY Therapeutic Area (USD Billion)
-
\r\n\r\n\r\nHIV
-
\r\n\r\n\r\nHepatitis
-
\r\n\r\n\r\nInfluenza
-
\r\n\r\n\r\nHerpes
-
Simplex Virus
-
\r\n\r\n\r\n\r\n\r\nAntiviral Drugs
-
Market, BY Route of Administration (USD Billion)
-
\r\n\r\n\r\nOral
-
\r\n\r\n\r\nInjectable
-
\r\n\r\n\r\nTopical
-
\r\n\r\n\r\n\r\n\r\nAntiviral
-
Drugs Market, BY Distribution Channel (USD Billion)
-
\r\n\r\n\r\nHospital
-
Pharmacy
-
\r\n\r\n\r\nRetail Pharmacy
-
\r\n\r\n\r\nOnline
-
Pharmacy
-
\r\n\r\n\r\n\r\n\r\nAntiviral Drugs Market,
-
BY Regional (USD Billion)
-
\r\n\r\n\r\nNorth America
-
\r\n\r\n\r\nUS
-
\r\n\r\n\r\nCanada
-
\r\n\r\n\r\n\r\n\r\nEurope
-
\r\n\r\n\r\nGermany
-
\r\n\r\n\r\nUK
-
\r\n\r\n\r\nFrance
-
\r\n\r\n\r\nRussia
-
\r\n\r\n\r\nItaly
-
\r\n\r\n\r\nSpain
-
\r\n\r\n\r\nRest
-
of Europe
-
\r\n\r\n\r\n\r\n\r\nAPAC
-
\r\n\r\n\r\nChina
-
\r\n\r\n\r\nIndia
-
\r\n\r\n\r\nJapan
-
\r\n\r\n\r\nSouth
-
Korea
-
\r\n\r\n\r\nMalaysia
-
\r\n\r\n\r\nThailand
-
\r\n\r\n\r\nIndonesia
-
\r\n\r\n\r\nRest
-
of APAC
-
\r\n\r\n\r\n\r\n\r\nSouth America
-
\r\n\r\n\r\nBrazil
-
\r\n\r\n\r\nMexico
-
\r\n\r\n\r\nArgentina
-
\r\n\r\n\r\nRest
-
of South America
-
\r\n\r\n\r\n\r\n\r\nMEA
-
\r\n\r\n\r\nGCC
-
Countries
-
\r\n\r\n\r\nSouth Africa
-
\r\n\r\n\r\nRest
-
of MEA
-
\r\n\r\n\r\n\r\n\r\n\r\n\r\n
-
\r\n
-
\r\n\r\n\r\nCompetitive
-
Landscape
-
\r\n\r\n\r\nOverview
-
\r\n\r\n\r\nCompetitive
-
Analysis
-
\r\n\r\n\r\nMarket share Analysis
-
\r\n\r\n\r\nMajor
-
Growth Strategy in the Antiviral Drugs Market
-
\r\n\r\n\r\nCompetitive
-
Benchmarking
-
\r\n\r\n\r\nLeading Players in Terms of Number of Developments
-
in the Antiviral Drugs Market
-
\r\n\r\n\r\nKey developments and growth
-
strategies
-
\r\n\r\n\r\nNew Product Launch/Service Deployment
-
\r\n\r\n\r\nMerger
-
& Acquisitions
-
\r\n\r\n\r\nJoint Ventures
-
\r\n\r\n\r\n\r\n\r\nMajor
-
Players Financial Matrix
-
\r\n\r\n\r\nSales and Operating Income
-
\r\n\r\n\r\nMajor
-
Players R&D Expenditure. 2023
-
\r\n\r\n\r\n\r\n\r\n\r\n\r\nCompany
-
Profiles
-
\r\n\r\n\r\nMerck and Co
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nPfizer
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nAbbVie
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nGilead Sciences
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nRoche
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nJohnson and Johnson
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nTeva Pharmaceutical Industries
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nNovartis
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nMylan
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nDr. Reddy's Laboratories
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nBayer
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nBristolMyers Squibb
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nHoffmannLa Roche
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nAstraZeneca
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nGlaxoSmithKline
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\n\r\n\r\nAppendix
-
\r\n\r\n\r\nReferences
-
\r\n\r\n\r\nRelated
-
Reports
-
\r\n\r\n\r\n\r\n\r\nLIST Of tables
-
\r\n
-
\r\n\r\n\r\nLIST
-
OF ASSUMPTIONS
-
\r\n\r\n\r\nNorth America Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nNorth
-
America Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA,
-
\r\n\r\n\r\nNorth America Antiviral Drugs
-
Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nNorth America Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nNorth
-
America Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nUS Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUS
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nUS Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUS
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nUS Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nCanada
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nCanada Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nCanada
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nCanada Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nCanada
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nEurope Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nEurope
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nEurope Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nEurope
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nEurope Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGermany
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nGermany Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGermany
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nGermany Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGermany
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nUK Antiviral Drugs Market SIZE ESTIMATES &
-
FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUK Antiviral
-
Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nUK Antiviral Drugs Market SIZE ESTIMATES &
-
FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUK
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nUK Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nFrance
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nFrance Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nFrance
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nFrance Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nFrance
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nRussia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRussia
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRussia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRussia
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRussia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nItaly
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nItaly Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nItaly
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nItaly Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nItaly
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nSpain Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSpain
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSpain Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSpain
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSpain Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of Europe Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of Europe Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of Europe Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nRest of Europe Antiviral Drugs
-
Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of Europe Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nAPAC Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nAPAC
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nAPAC Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nAPAC
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nAPAC Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nChina
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nChina Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nChina
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nChina Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nChina
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nIndia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nIndia
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nIndia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nIndia
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nIndia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nJapan
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nJapan Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nJapan
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nJapan Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nJapan
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nSouth Korea Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Korea Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA,
-
\r\n\r\n\r\nSouth Korea Antiviral Drugs
-
Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nSouth Korea Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Korea Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nMalaysia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMalaysia
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nMalaysia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMalaysia
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nMalaysia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nThailand
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nThailand Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nThailand
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nThailand Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nThailand
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nIndonesia Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nIndonesia
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nIndonesia Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nIndonesia
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nIndonesia Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of APAC Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of APAC Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of APAC Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nRest of APAC Antiviral Drugs
-
Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of APAC Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSouth America Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
America Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA,
-
\r\n\r\n\r\nSouth America Antiviral Drugs
-
Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nSouth America Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
America Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nBrazil Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nBrazil
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nBrazil Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nBrazil
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nBrazil Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMexico
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nMexico Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMexico
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nMexico Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMexico
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nArgentina Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nArgentina
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nArgentina Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nArgentina
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nArgentina Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of South America Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE,
-
\r\n\r\n\r\nRest of South America Antiviral
-
Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nRest of South America Antiviral Drugs Market
-
SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of South America Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION
-
CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest of South America
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nMEA Antiviral Drugs Market SIZE ESTIMATES &
-
FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMEA
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nMEA Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMEA
-
Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nMEA Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGCC
-
Countries Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nGCC Countries Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY THERAPEUTIC AREA, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGCC
-
Countries Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION,
-
\r\n\r\n\r\nGCC Countries Antiviral Drugs
-
Market SIZE ESTIMATES & FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGCC
-
Countries Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSouth Africa Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Africa Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA,
-
\r\n\r\n\r\nSouth Africa Antiviral Drugs
-
Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nSouth Africa Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Africa Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of MEA Antiviral Drugs Market SIZE
-
ESTIMATES & FORECAST, BY DRUG TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of MEA Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY THERAPEUTIC AREA,
-
\r\n\r\n\r\nRest of MEA Antiviral Drugs
-
Market SIZE ESTIMATES & FORECAST, BY ROUTE OF ADMINISTRATION, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nRest of MEA Antiviral Drugs Market SIZE ESTIMATES
-
& FORECAST, BY DISTRIBUTION CHANNEL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of MEA Antiviral Drugs Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nPRODUCT LAUNCH/PRODUCT DEVELOPMENT/APPROVAL
-
\r\n\r\n\r\nACQUISITION/PARTNERSHIP
-
\r\n\r\n\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\n
-
\r\nLIST
-
Of figures
-
\r\n
-
\r\n\r\n\r\nMARKET SYNOPSIS
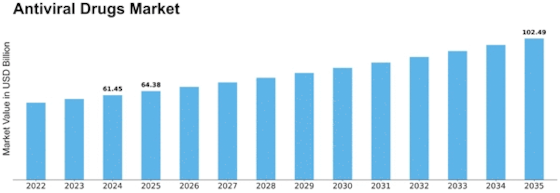
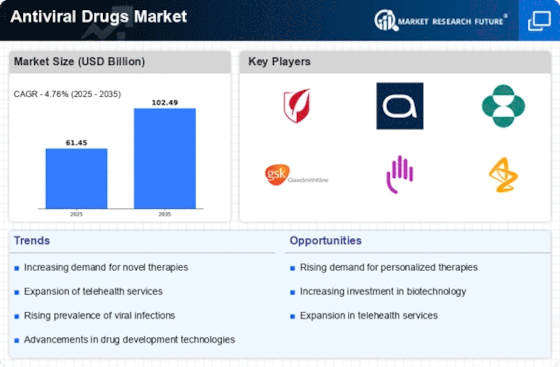
-
\r\n\r\n\r\nNORTH
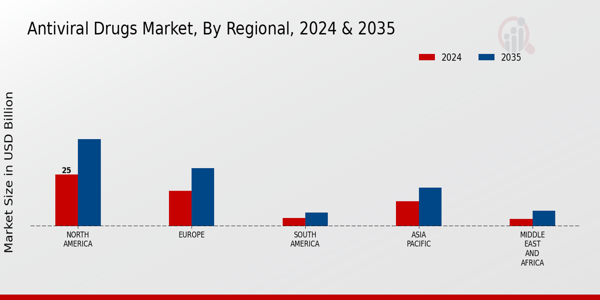
-
AMERICA ANTIVIRAL DRUGS MARKET ANALYSIS
-
\r\n\r\n\r\nUS ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nUS ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nUS ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nUS ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nUS ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nCANADA ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nCANADA ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nCANADA ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nCANADA ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nCANADA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nEUROPE ANTIVIRAL
-
DRUGS MARKET ANALYSIS
-
\r\n\r\n\r\nGERMANY ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nGERMANY ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nGERMANY ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nGERMANY ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nGERMANY
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nUK ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nUK ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nUK ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nUK ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nUK ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nFRANCE ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nFRANCE ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nFRANCE ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nFRANCE ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nFRANCE
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nRUSSIA ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nRUSSIA ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nRUSSIA ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nRUSSIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nRUSSIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nITALY ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nITALY ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nITALY ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nITALY ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nITALY ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nSPAIN ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nSPAIN ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nSPAIN ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nSPAIN ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nSPAIN ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nREST OF EUROPE ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nREST OF EUROPE ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nREST OF EUROPE
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nREST
-
OF EUROPE ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nREST
-
OF EUROPE ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nAPAC
-
ANTIVIRAL DRUGS MARKET ANALYSIS
-
\r\n\r\n\r\nCHINA ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nCHINA ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nCHINA ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nCHINA ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nCHINA ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nINDIA ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nINDIA ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nINDIA ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nINDIA ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nINDIA ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nJAPAN ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nJAPAN ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nJAPAN ANTIVIRAL DRUGS MARKET
-
ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nJAPAN ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nJAPAN ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nSOUTH KOREA ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nSOUTH KOREA ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nSOUTH KOREA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nSOUTH
-
KOREA ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nSOUTH
-
KOREA ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nMALAYSIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nMALAYSIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nMALAYSIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nMALAYSIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nMALAYSIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nTHAILAND
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nTHAILAND
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nTHAILAND
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nTHAILAND
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nTHAILAND
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nINDONESIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nINDONESIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nINDONESIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nINDONESIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nINDONESIA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nREST OF APAC
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nREST OF
-
APAC ANTIVIRAL DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nREST
-
OF APAC ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nREST
-
OF APAC ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nREST
-
OF APAC ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nSOUTH
-
AMERICA ANTIVIRAL DRUGS MARKET ANALYSIS
-
\r\n\r\n\r\nBRAZIL ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nBRAZIL ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nBRAZIL ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nBRAZIL
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nBRAZIL
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nMEXICO ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nMEXICO ANTIVIRAL DRUGS
-
MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nMEXICO ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nMEXICO
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nMEXICO
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nARGENTINA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nARGENTINA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nARGENTINA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nARGENTINA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nARGENTINA
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nREST OF SOUTH
-
AMERICA ANTIVIRAL DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nREST
-
OF SOUTH AMERICA ANTIVIRAL DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nREST
-
OF SOUTH AMERICA ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nREST
-
OF SOUTH AMERICA ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nREST
-
OF SOUTH AMERICA ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nMEA
-
ANTIVIRAL DRUGS MARKET ANALYSIS
-
\r\n\r\n\r\nGCC COUNTRIES ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nGCC COUNTRIES ANTIVIRAL
-
DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nGCC COUNTRIES
-
ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nGCC
-
COUNTRIES ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nGCC
-
COUNTRIES ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nSOUTH
-
AFRICA ANTIVIRAL DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nSOUTH
-
AFRICA ANTIVIRAL DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nSOUTH
-
AFRICA ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nSOUTH
-
AFRICA ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nSOUTH
-
AFRICA ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nREST
-
OF MEA ANTIVIRAL DRUGS MARKET ANALYSIS BY DRUG TYPE
-
\r\n\r\n\r\nREST
-
OF MEA ANTIVIRAL DRUGS MARKET ANALYSIS BY THERAPEUTIC AREA
-
\r\n\r\n\r\nREST
-
OF MEA ANTIVIRAL DRUGS MARKET ANALYSIS BY ROUTE OF ADMINISTRATION
-
\r\n\r\n\r\nREST
-
OF MEA ANTIVIRAL DRUGS MARKET ANALYSIS BY DISTRIBUTION CHANNEL
-
\r\n\r\n\r\nREST
-
OF MEA ANTIVIRAL DRUGS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nKEY
-
BUYING CRITERIA OF ANTIVIRAL DRUGS MARKET
-
\r\n\r\n\r\nRESEARCH PROCESS
-
OF MRFR
-
\r\n\r\n\r\nDRO ANALYSIS OF ANTIVIRAL DRUGS MARKET
-
\r\n\r\n\r\nDRIVERS
-
IMPACT ANALYSIS: ANTIVIRAL DRUGS MARKET
-
\r\n\r\n\r\nRESTRAINTS IMPACT
-
ANALYSIS: ANTIVIRAL DRUGS MARKET
-
\r\n\r\n\r\nSUPPLY / VALUE CHAIN:
-
ANTIVIRAL DRUGS MARKET
-
\r\n\r\n\r\nANTIVIRAL DRUGS MARKET, BY DRUG
-
TYPE, 2025 (% SHARE)
-
\r\n\r\n\r\nANTIVIRAL DRUGS MARKET, BY DRUG
-
TYPE, 2019 TO 2035 (USD Billions)
-
\r\n\r\n\r\nANTIVIRAL DRUGS MARKET,
-
BY THERAPEUTIC AREA, 2025 (% SHARE)
-
\r\n\r\n\r\nANTIVIRAL DRUGS MARKET,
-
BY THERAPEUTIC AREA, 2019 TO 2035 (USD Billions)
-
\r\n\r\n\r\nANTIVIRAL
-
DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2025 (% SHARE)
-
\r\n\r\n\r\nANTIVIRAL
-
DRUGS MARKET, BY ROUTE OF ADMINISTRATION, 2019 TO 2035 (USD Billions)
-
\r\n\r\n\r\nANTIVIRAL
-
DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2025 (% SHARE)
-
\r\n\r\n\r\nANTIVIRAL
-
DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2019 TO 2035 (USD Billions)
-
\r\n\r\n\r\nANTIVIRAL
-
DRUGS MARKET, BY REGIONAL, 2025 (% SHARE)
-
\r\n\r\n\r\nANTIVIRAL DRUGS
-
MARKET, BY REGIONAL, 2019 TO 2035 (USD Billions)
-
\r\n\r\n\r\nBENCHMARKING
-
OF MAJOR COMPETITORS
-
\r\n\r\n\r\n








Leave a Comment