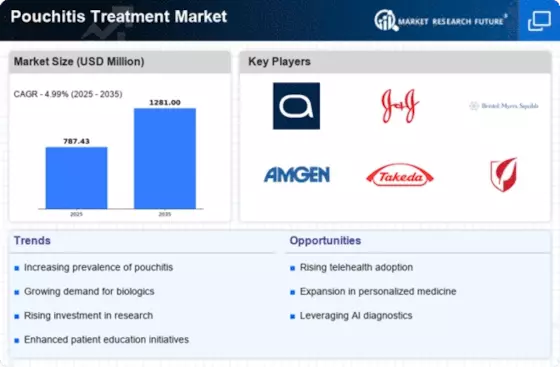
Top Industry Leaders in the America Pouchitis Treatment Market
 Latest America Pouchitis Treatment Companies Update
Latest America Pouchitis Treatment Companies Update
-
Mar 2023: The good results from the Phase 4 EARNEST research into vedolizumab for treating chronic pouchitis have been released by the New England Journal of Medicine (NEJM), with Takeda Canada Inc. being glad to announce this. The article published in the NEJM is headed "Vedolizumab for the Treatment of Chronic Pouchitis." After an ileal pouch (ileal pouch-anal anastomosis, or IPAA) is surgically created to help those with ulcerative colitis retain stool, patients may develop pouchitis. Bleeding, stomach pain, and fecal incontinence are all possible effects of pouchitis. About one-fifth of these patients may develop chronic pouchitis, characterized by symptoms lasting longer than four weeks. Patients on vedolizumab showed better clinical response compared to placebo at Weeks 14 and 34, in addition to mPDAI remission.
-
Mar 2023: To maintain the progress of its chronic pouchitis treatment, Applied Molecular Transport made significant sacrifices last year, including the layoff of 40% of its workforce and early-stage pipeline operations. The company is still looking for a partner to help the candidate enter phase 3 trials, even though they have lost the excess weight. The medication is an oral, gut-specific fusion protein of interleukin-1 (IL-1), known as AMT-101. With ulcerative colitis patients experiencing an inferior clinical remission rate from AMT-101 compared to the placebo cohort, the company concluded the previous year in financial distress. This came after an unsuccessful attempt to use the therapy in the same indication versus Humira in July. In the pouchitis indication, where AMT was designated as an orphan medication by the FDA in November, the company has experienced more success. Pouchitis is an infection that can happen after colon surgery and for which there aren't any FDA-approved medications.
List of America Pouchitis Treatment Key companies in the market
- Pfizer
- Abbott
- Allergan Plc
- Bayer
- Takeda Pharmaceutical Company Limited
- Alfa sigma
- Atlantic Healthcare
- Tillotts Pharma AG
- AstraZeneca
- Novartis AG
- Teva Pharmaceutical Industries Ltd.
- Sanofi
- B. Braun Medical Inc.
- Valeant Pharmaceuticals
- Sucampo Pharmaceuticals
- Ironwood Pharmaceuticals, Inc.
- Astellas Pharma Inc.
- Synergy Pharmaceuticals, Inc.
- Ardelyx, Inc.
- Sebela Pharmaceuticals