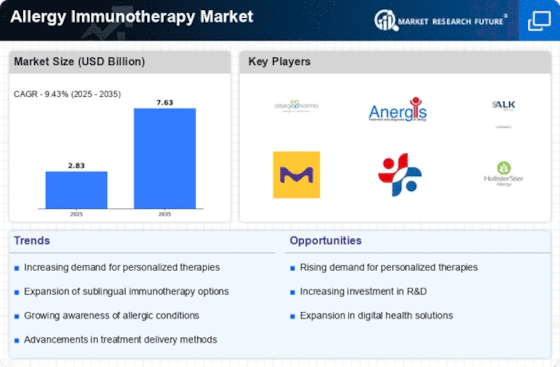
Top Industry Leaders in the Allergy immunotherapy Market
 Latest Allergy Immunotherapy Companies Update
Latest Allergy Immunotherapy Companies Update
-
Oct 2023: Following Intrommune Therapeutics' completion of the final patient visit, a trial examining desensitization immunotherapy administered as toothpaste for adults with peanut allergy achieved its ultimate milestone. Thirty-two people with peanut allergies were divided into two groups for the Phase I OMEGA clinical trial (NCT04603300), a multi-center, randomized, placebo-controlled, double-blind research. Each group was assigned to receive either a placebo or an increasing dose of the company's candidate, INT301. The safety of the medication in comparison to a placebo is the main result. The frequency of adverse responses and the proportion of participants tolerating the most medication are used to gauge safety. The medication is delivered in fully functional toothpaste by Intrommune Therapeutics' candidate, using the company's oral mucosal immunotherapy (OMIT) technology. OMIT's straightforward administration promotes better adherence, crucial for desensitizing a patient to a specific allergy. Allergy immunotherapy requires consistent exposure.
-
June 2023: Results from a phase 3 trial showed that young patients with allergic rhinitis from house dust mites (HDM) responded favorably to ALK's sublingual allergy immunotherapy tablet. Currently, Odactra is approved for the treatment of HDM-induced allergic rhinitis, regardless of the presence of conjunctivitis, if the condition is verified by positive skin testing to authorized house dust mite allergen extracts in patients 12 years of age and older or by positive in vitro testing for IgE antibodies to Dermatophagoides farinae and Dermatophagoides pteronyssinus house dust mites. The company is anticipated to work with regulatory bodies to go after an expansion of the drug's indication considering these study results.
List of Allergy immunotherapy Key companies in the market
- ALK Abello (U.K.)
- Allergy Therapeutics (U.K.)
- Circassia (U.K.)
- DVB Technologies (U.K.)
- HAL Allergy Group (U.K.)
- Merck KGaA Allergopharma (U.K.)
- Stallergenes Greer (U.K.)