Summary Overview
Drug Development Biometrics Services Market Overview
The global Drug Development Biometrics Services market is experiencing significant growth due to the increasing demand for clinical trials, advancements in biotechnology, and the growing complexity of regulatory requirements. Biometrics services, which include data analysis, statistical modelling, and clinical trial management, play a crucial role in the drug development lifecycle. With the rise of precision medicine, the demand for data-driven insights and robust analytics is surging. This market benefits from both domestic and international investments in drug development and clinical research.
Our report offers an in-depth analysis of emerging procurement trends, identifying cost-saving opportunities through strategic sourcing, vendor management, and advanced technology integration. We also highlight the challenges faced in the drug development process, including data quality, regulatory compliance, and market access. Furthermore, the report emphasizes the importance of digital procurement tools and analytics in accurately forecasting market needs to assist clients in staying ahead in this dynamic and competitive landscape. Strategic sourcing and procurement management are essential to streamline the procurement process and maintain cost-effective solutions. As competition intensifies, pharmaceutical companies are leveraging market intelligence and procurement analytics to optimize their supply chain management and drive efficiency in drug development biometrics services.
The outlook for the drug development biometrics services market is promising, with projections indicating robust growth through 2032:
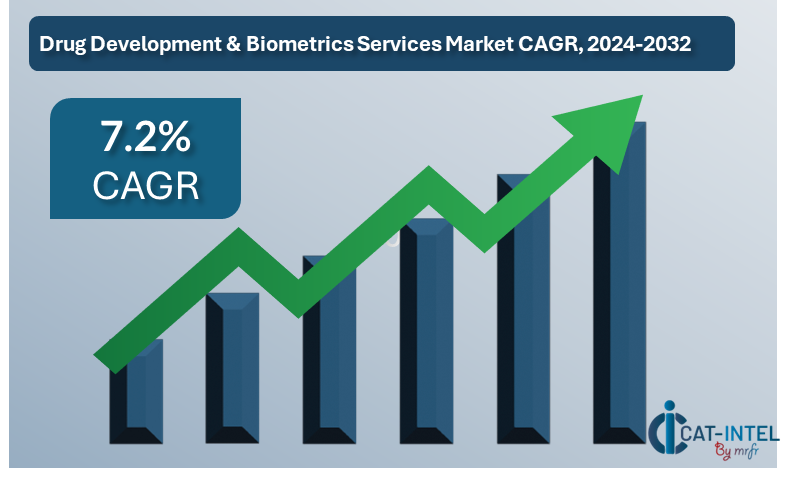
Market Size: The global drug development biometrics services market is expected to reach approximately USD 12.4 billion by 2032, reflecting a compound annual growth rate (CAGR) of around 7.2% from 2024 to 2032.
Sector Contributions:Growth is primarily driven by:
Pharmaceutical and Biotechnology Industry: The increasing complexity of clinical trials, rising demand for data-driven insights, and need for faster drug approval processes are driving the demand for biometrics services.
Contract Research Organizations (CROs): The expanding role of CROs in managing clinical trials and providing analytics services is contributing significantly to the market growth.
Technological Transformation and Innovations: Advances in artificial intelligence (AI), machine learning, and big data analytics are improving the accuracy and efficiency of clinical data analysis. Additionally, the growing need for real-time analytics and cloud-based solutions is enhancing the flexibility and scalability of biometrics services.
Funding Initiatives: Increased investment in clinical trial research and development (R&D), as well as funding for regulatory-compliant technologies, is driving the growth of the drug development biometrics services market.
Regional Insights: North America, particularly the United States, leads the market, driven by a high number of clinical trials and regulatory advancements. The Asia-Pacific region is expected to see rapid growth due to an increasing number of clinical trials and improving healthcare infrastructure.
Key Trends and Sustainability Outlook
Sustainability Initiatives: The focus on minimizing clinical trial costs and reducing the environmental footprint of drug development is increasing. Adoption of virtual trials and decentralized clinical trials (DCTs) is making the process more efficient and sustainable.
Health and Regulatory Trends: Stringent regulatory requirements and a rising emphasis on patient-centric clinical trials are influencing demand for high-quality biometrics services. Companies are increasingly focusing on precision medicine and personalized treatment strategies.
Digital Transformation: The integration of digital tools, such as cloud-based data management platforms, blockchain for data security, and AI for data analysis, is accelerating the transformation of drug development processes.
Growth Drivers:
Health Benefits and Precision Medicine: The growing demand for personalized treatments is driving the need for more advanced biometrics services in drug development.
Global Pharmaceutical Market Growth: The expanding global pharmaceutical market, especially in emerging markets, is fuelling the demand for more robust and scalable biometrics solutions.
Regulatory Demands and Compliance: Increasingly stringent regulatory requirements for clinical trials are driving the need for more accurate and efficient biometrics services.
Digital Transformation in Clinical Trials: The increasing use of digital tools for data analysis, cloud computing, and AI integration is enhancing the efficiency and accuracy of drug development biometrics services.
Overview of Market Intelligence Services for the Drug Development Biometrics Services Market
Recent analysis indicates fluctuations in pricing and demand for biometrics services due to variable drug development cycles and complex regulatory environments. Market reports offer detailed cost forecasts and procurement strategies that help stakeholders manage cost volatility, mitigate risks, and ensure high-quality service providers. By leveraging insights from these reports, pharmaceutical companies can effectively navigate pricing challenges while ensuring access to world-class biometrics services. Strategic sourcing practices will enable stakeholders to streamline the procurement process and gain a competitive edge in the market.
Procurement Intelligence for Drug Development Biometrics Services Market: Category Management and Strategic Sourcing
"To remain competitive in the drug development biometrics services market, companies are optimizing procurement strategies, leveraging spend analysis solutions for vendor performance evaluation, and enhancing supply chain efficiency through supply market intelligence. Procurement category management and strategic sourcing are essential in achieving cost-effective procurement and ensuring the timely availability of high-quality biometrics services for drug development."
Pricing Outlook for Drug Development Biometrics Services Market: Spend Analysis
The drug development biometrics services market is navigating a complex pricing environment driven by various factors such as advancements in clinical trial technologies, increased demand for personalized treatments, and regulatory pressures. This dynamic pricing environment reflects the evolving landscape of drug development, particularly the demand for high-quality data analysis and real-time insights in clinical trials.
Line chart illustrating the pricing outlook for the drug development biometrics services industry from 2022 to 2032.Our advanced analysis shows a steady upward trajectory in biometrics services pricing, driven by several key factors, including:
Rising Labor and Technology Costs: The increasing complexity of clinical trials, the demand for skilled professionals, and the integration of advanced technologies like AI and machine learning in data analysis are driving up labor and technology costs.
Surge in Demand for Precision Medicine: The rise of personalized medicine and the demand for customized treatment options are increasing the need for biometrics services, contributing to higher prices.
Regulatory Pressures and Compliance: Stricter regulatory requirements for clinical trials, particularly in data management and reporting, are pushing service providers to enhance quality and accuracy, resulting in higher service costs.
Market Competition and Vendor Specialization: With the rise of specialized contract research organizations (CROs) and the increasing use of decentralized clinical trials, the competitive pricing pressures are creating variability in the market. This leads to price volatility, particularly as companies aim to secure partnerships with top-tier service providers.
Technological Advancements: The growing integration of cloud-based solutions and AI-driven analytics in drug development is increasing the demand for sophisticated biometrics services, leading to higher prices for high-quality services.
Cost Breakdown for Drug Development Biometrics Services Market: Cost Saving Opportunities
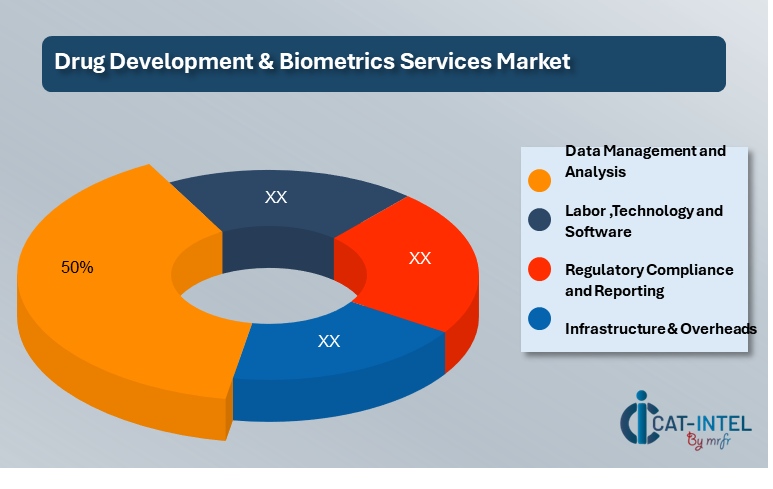
-
Data Management and Analysis (50%)
Description: This represents the bulk cost of data processing, including data collection, cleaning, analysis, and management of clinical trial data. It involves the use of specialized software, cloud storage, and AI tools to ensure the accuracy and efficiency of data handling.
Trends: As clinical trials become more complex, the demand for high-quality data management increases. The trend is shifting towards cloud-based solutions and AI-driven analytics, which optimize data analysis while reducing manual efforts. Costs may rise as advanced technologies are integrated into services, but long-term savings can be achieved through automation and increased efficiency in data processing.
Labor (XX%)
Technology and Software (XX%)
Regulatory Compliance and Reporting (XX%)
Infrastructure & Overheads (XX%)
Cost Saving Opportunities: Negotiation Lever and Purchasing Negotiation Strategies for Drug Development Biometrics Services Market
In the drug development biometrics services market, optimizing procurement strategies can lead to significant cost savings and operational efficiency. Collaborative partnerships with clinical research organizations (CROs) allow pharmaceutical companies to negotiate better pricing on clinical trials, data collection, and analysis services. Leveraging economies of scale by bundling services such as data management, biometrics analysis, and regulatory reporting can result in volume-based discounts.

Supply and Demand Overview of the Drug Development Biometrics Services Market: Demand-Supply Dynamics and Buyer Intelligence for Effective Supplier Relationship Management (SRM)
The drug development biometrics services market is experiencing growth driven by the increasing complexity of clinical trials, regulatory requirements, and advancements in biotechnology. The demand is strong in areas like clinical data management, biostatistics, and regulatory submissions, as pharmaceutical companies look to streamline drug development processes and meet stringent standards.
Demand Factors:
Regulatory Pressures: As the pharmaceutical industry faces stricter regulations globally, there is an increasing need for high-quality biometrics services to ensure compliance and accurate reporting in clinical trials.
Biotechnology Advancements: New drug development technologies and the rise of personalized medicine drive demand for sophisticated data analytics and biostatistical services to analyse clinical trial data effectively.
Outsourcing Trends: Pharmaceutical companies are increasingly outsourcing biometrics and data management services to contract research organizations (CROs) to reduce costs and expedite drug development timelines.
Rising Global Healthcare Needs: A growing global population and increasing healthcare needs lead to an uptick in the number of clinical trials, boosting demand for biometrics services related to drug development.
Supply Factors:
CRO Market Growth: The growth of contract research organizations (CROs) as key players in the market has led to a stable supply of biometrics services to support pharmaceutical companies' clinical trial needs.
Technological Advancements: Innovations in cloud computing, artificial intelligence, and machine learning are enhancing data management and analysis capabilities, improving service quality and efficiency.
Skilled Labor Availability: The availability of skilled professionals in data science, biostatistics, and regulatory affairs supports the growing demand for high-quality biometrics services.
Global Competition: Intense competition among service providers in the biometrics space drives continuous improvements in service offerings, pricing, and operational efficiency.
Regional Demand-Supply Outlook: Drug Development Biometrics Services Market
The image shows growing demand for Drug Development Biometrics Services in North America and Europe, with increasing competition and demand for specialized services.
North America: A Key Player in the Drug Development Biometrics Services Market North America, particularly the U.S., plays a crucial role in the biometrics services market, characterized by:
Leading Providers: North American contract research organizations (CROs) are leaders in providing biometrics services, offering advanced data management and statistical services for clinical trials.
Strong Regulatory Environment: The U.S. has stringent regulatory frameworks, ensuring the need for high-quality biometrics services to ensure compliance with FDA and other international standards.
Biotechnology Hub: The presence of numerous biotech firms and pharmaceutical companies in North America fuels demand for biometrics services, particularly in drug development, clinical trials, and data analytics.
Technological Innovation: North American companies are investing in AI, machine learning, and cloud technologies to enhance biometrics service offerings and streamline clinical trial processes.
Consumer Trends: Increased focus on personalized medicine and precision healthcare accelerates the demand for specialized biometrics services to analyse and interpret clinical trial data effectively.
North America remains a key hub for Drug Development Biometrics market and its growth
Supplier Landscape: Supplier Negotiations and Strategies for Drug Development Biometrics Services Market
The Drug Development Biometrics Services market features a dynamic and evolving supplier landscape, comprising both global and specialized regional players. These suppliers provide critical services such as data management, statistical analysis, clinical trial management, regulatory compliance, and advanced analytics to support the development and approval of pharmaceuticals.
Key suppliers in this market include contract research organizations (CROs), technology providers, and analytics companies. Collaboration between these suppliers and pharmaceutical companies is vital for optimizing clinical trials, ensuring compliance, and accelerating the drug development process.
Some of the key suppliers in the Drug Development Biometrics Services market include:
Parexel International
Covance (LabCorp Drug Development)
IQVIA
Medidata Solutions (Dassault Systems)
PPD (Pharmaceutical Product Development)
WuXi AppTec
Charles River Laboratories
Celerion
Bioclinica
Syneos Health
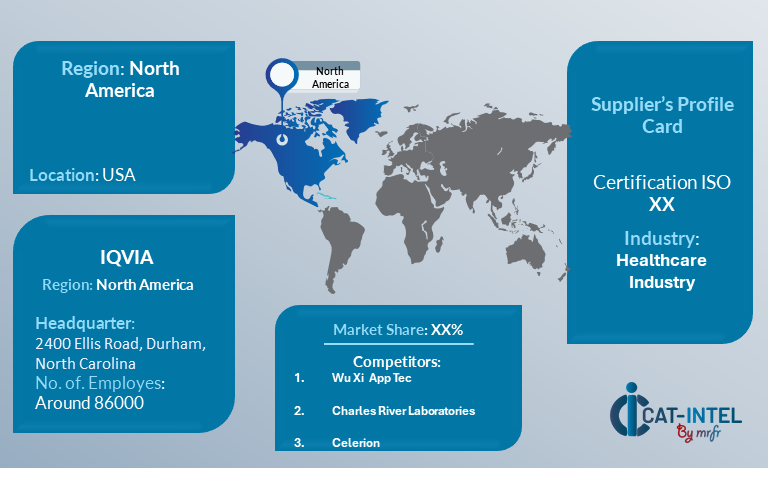
Key Development: Procurement Category significant development
| Procurement Category | Significant Development |
| Data Management Services | Growth in the use of cloud-based platforms for real-time data sharing and analysis, enhancing collaboration. |
| Clinical Trial Management | Increasing reliance on decentralized and virtual clinical trials, improving patient recruitment and data quality. |
| Statistical Analysis Services | Advancements in AI and machine learning for more precise and faster statistical analysis in clinical trials. |
| Regulatory Compliance | Enhanced integration of regulatory technology to ensure faster submission processes and better compliance. |
| Technology Solutions | Rising adoption of AI, big data analytics, and real-time monitoring to optimize trial design and performance. |
| Procurement Attribute/Metric | Details |
| Market Sizing | The Drug Development Biometrics Services market is projected to grow from USD 8.4 billion in 2023 to USD 12.4 billion by 2032, with a CAGR of 7.2% during the forecast period. |
| Adoption of Biometrics in Clinical Trials | The integration of biometrics in clinical trials is growing rapidly, with a focus on patient-centric data collection and real-time monitoring, especially in clinical trial management and pharmacovigilance. |
| Top Strategies for 2024 | Emphasis on digital transformation, including AI and machine learning, for more efficient biometrics data collection and analysis, adoption of decentralized clinical trials, and partnerships with CROs for cost-effective solutions. |
| Automation in Drug Development | More than 35% of drug developers are adopting automation technologies for data management, analysis, and clinical trial monitoring to improve accuracy and reduce time to market. |
| Procurement Challenges | Key challenges include regulatory compliance complexities, data privacy concerns, reliance on external CROs, and increasing competition for specialized biometrics talent. |
| Key Suppliers | Leading providers include Parexel International, LabCorp Drug Development, IQVIA, and Covance, which are offering specialized biometrics services such as statistical analysis, data management, and clinical trial monitoring. |
| Key Regions Covered | Major markets include North America, Europe, and Asia-Pacific, with significant growth in the U.S., Europe, and emerging markets in China and India. |
| Market Drivers and Trends | Growth is driven by increased outsourcing of clinical trials, advancements in AI for biometrics analysis, the rise of personalized medicine, and the growing need for efficient regulatory compliance. |










