Summary Overview
DNA Vaccines Market Overview:
The global DNA vaccines market is experiencing significant growth, driven by advancements in biotechnology, increasing demand for effective immunization solutions, and the rise of novel infectious diseases. This market includes vaccines targeting various diseases, including viral infections, cancer, and genetic disorders. Our report provides a detailed analysis of procurement trends, emphasizing cost optimization strategies and leveraging digital tools to streamline research, development, and distribution processes.
Key challenges in procurement include managing the high costs of production, ensuring cold chain stability, and addressing stringent regulatory requirements. Digital procurement tools and strategic sourcing are critical for optimizing the vaccine supply chain and enhancing long-term competitiveness. As global demand continues to rise, companies are utilizing market intelligence to improve efficiency and minimize risks.
The DNA vaccines market is expected to maintain robust growth through 2032, with key highlights including:
-
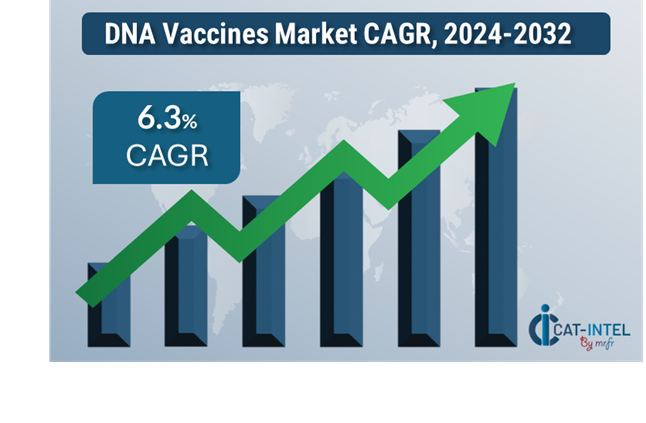
Market Size: The global DNA vaccines market is projected to reach USD 774.43billion by 2032, growing at a CAGR of approximately 6.3 % from 2024 to 2032.
-
Sector Contributions: Growth in the market is driven by: -
Healthcare Advancements: Increased investment in genetic and immunological research, driving the demand for innovative vaccines. -
Public Health Initiatives: Expanding immunization programs globally to combat emerging infectious diseases and strengthen population immunity. -
Technological Transformation and Innovations: Breakthroughs in DNA plasmid delivery systems, enhanced efficacy, and reduced production timelines are transforming vaccine development. -
Investment Initiatives: Pharmaceutical companies and biotech firms are investing in advanced production technologies, such as bioreactors and automated formulation systems, to enhance efficiency and reduce costs. -
Regional Insights: North America is a key contributor due to its robust biotech infrastructure, significant R&D investments, and supportive regulatory frameworks.
Key Trends and Sustainability Outlook:
-
Digital Transformation: Automation in vaccine development and manufacturing is improving scalability, reducing errors, and optimizing workflows. -
Advanced Delivery Mechanisms: Innovative delivery methods such as electroporation and needle-free injectors enhance vaccine uptake and efficacy. -
Focus on Sustainability: Greater emphasis on sustainable production methods, such as reducing energy consumption and minimizing waste in manufacturing processes. - Customization Trends: Growing demand for vaccines tailored to specific populations, age groups, and disease profiles.
-
Data-Driven R&D: Leveraging big data and AI to optimize vaccine design, improve trial outcomes, and ensure regulatory compliance.
Growth Drivers:
-
Rising Prevalence of Infectious Diseases: The ongoing emergence of infectious diseases necessitates the development of novel vaccine solutions. -
Cancer Immunotherapy: Increasing interest in DNA vaccines as a tool for personalized cancer treatment. -
Technological Advancements: Innovations in synthetic biology and molecular engineering are boosting the production of high-efficacy vaccines. -
Regulatory Frameworks: Supportive policies and expedited approval pathways are accelerating vaccine availability. -
Global Immunization Goals: Collaborative efforts by governments and international organizations to increase vaccine access and coverage.
Overview of Market Intelligence Services for the DNA Vaccines Market:
Recent analyses have identified key challenges such as high production costs, complex regulatory pathways, and the need for cold chain logistics. Market intelligence reports provide actionable insights into procurement opportunities, helping companies identify cost-saving measures, optimize vendor management, and enhance supply chain resilience. These reports also assist organizations in maintaining compliance with stringent regulations and upholding quality standards while effectively managing expenses.
Procurement Intelligence for DNA Vaccines: Category Management and Strategic Sourcing:
To remain competitive in the DNA vaccines market, companies are enhancing procurement processes by employing spend analysis for supplier tracking and improving supply chain efficiency through market intelligence. Effective category management and strategic sourcing are crucial for reducing procurement costs and ensuring a consistent supply of high-quality vaccine materials. By utilizing actionable market intelligence, businesses can streamline procurement strategies, navigate regulatory complexities, and secure favourable terms to meet their vaccine production needs.

Pricing Outlook for DNA Vaccines: Spend Analysis
The pricing outlook for DNA vaccines is anticipated to remain moderately dynamic, influenced by several critical factors. Variations in raw material costs, such as synthetic DNA plasmids and reagents, fluctuating research and development expenses, and evolving regulatory requirements for vaccine approval and distribution significantly drive price trends. Additionally, the growing demand for innovative delivery systems and personalized vaccine solutions is contributing to upward pricing pressures.
Graph shows general upward trend pricing for DNA Vaccines and growing demand. However, there may be fluctuations influenced by economic conditions, technological advancements, and competitive dynamic.
Efforts to optimize procurement strategies, enhance supplier relationships, and adopt advanced biomanufacturing technologies are vital for cost control. Utilizing digital tools for market trend analysis, cost forecasting through data-driven insights, and efficient supply chain planning can further improve cost management.
Collaborating with reliable suppliers, securing long-term agreements, and streamlining production processes are essential strategies to manage pricing effectively. Despite challenges, maintaining vaccine efficacy, aligning with regulatory compliance, and leveraging advancements in biotechnology will be crucial to achieving cost efficiency.
Cost Breakdown for DNA Vaccines: Total Cost of Ownership (TCO) and Cost-Saving Opportunities

- Raw Materials (50%)
- Description: A significant portion of the cost in DNA vaccine production stems from raw materials, including synthetic DNA sequences, carrier molecules, and reagents. These costs are heavily influenced by global supply availability, production scalability, and regional sourcing dynamics.
- Trends: Companies are implementing strategies such as centralized procurement, long-term supplier contracts, and sourcing from local suppliers to mitigate raw material price volatility. The increased adoption of cost-effective and scalable synthetic methods is also influencing raw material pricing trends, particularly for high-purity components.
- Labor (XX%)
- Publishing Services (XX%)
- Infrastructure & Overheads (XX%)
Cost-Saving Opportunities: Negotiation Levers and Purchasing Negotiation Strategies
In the DNA vaccines market, optimizing procurement strategies and employing effective negotiation tactics can lead to substantial cost savings and operational improvements. Establishing long-term agreements with suppliers of key components, such as synthetic DNA sequences and delivery systems, can result in better pricing and favourable terms, including volume discounts and reduced shipping costs. Bulk procurement and forward contracts also provide opportunities to lock in lower rates and hedge against price volatility.
Collaborating with innovative sup pliers that emphasize scalability, and efficiency can offer additional benefits, such as access to advanced production technologies and cost reductions through streamlined processes. Leveraging digital tools for procurement, including demand forecasting and supplier performance tracking systems, enhances operational efficiency, reduces supply disruptions, and optimizes inventory levels. Diversifying supplier networks and implementing multi-supplier strategies mitigate risks such as material shortages or regulatory bottlenecks and provide greater leverage during negotiations.

Supply and Demand Overview for DNA Vaccines: Demand-Supply Dynamics and Buyer Intelligence for Effective Supplier Relationship Management (SRM)
The DNA vaccine market is witnessing robust growth, fuelled by increasing global demand for advanced immunization solutions. Supply and demand dynamics are shaped by factors such as technological advancements, production scalability, and regulatory requirements.
Demand Factors:
-
Global Health Initiatives: Rising demand for vaccines addressing emerging diseases and pandemic preparedness is driving the market. -
Personalized Medicine: Increasing interest in DNA-based immunotherapies tailored to individual needs is expanding the application of DNA vaccines. -
Technological Innovations: Advances in delivery mechanisms, such as electroporation devices, are increasing the adoption of DNA vaccines. -
Sustainability Goals: Growing emphasis on scalable, environmentally friendly vaccine production techniques is influencing procurement decisions.
Supply Factors:
-
Raw Material Availability: The supply of synthetic DNA plasmids, reagents, and delivery systems significantly affects production costs and timelines. -
Technological Advancements: Innovations in manufacturing processes, including automated plasmid synthesis and real-time quality monitoring, enhance supply reliability. -
Regulatory Frameworks: Compliance with stringent regulatory requirements impacts production scalability and timelines. -
Operational Efficiency: Investments in cutting-edge manufacturing facilities and streamlined logistics systems ensure consistent supply and reduced lead times.
Regional Demand-Supply Outlook: DNA Vaccines
Image shows growing demand for crates in both North America and Asia Pacific, with potential price increases and increased Competition.
North America: Dominance in DNA Vaccine
North America, led by the United States and Canada, is emerging as a powerhouse in the DNA vaccine market due to the following factors:
-
R&D Leadership: The region excels in vaccine research and development, supported by robust funding from government initiatives, private investors, and collaborations between biotech companies and academic institutions. -
Advanced Manufacturing Infrastructure: North America boasts state-of-the-art facilities equipped with the latest technologies, ensuring high-quality production and scalability. -
Regulatory Excellence: Strict adherence to regulatory standards, such as those set by the FDA and Health Canada, ensures the safety and efficacy of DNA vaccines, enhancing market credibility. -
Strong Market Demand: The prevalence of advanced healthcare systems and widespread immunization programs creates a consistent demand for cutting-edge vaccines. -
Focus on Innovation: North America is driving breakthroughs in vaccine delivery mechanisms, including novel electroporation techniques and nanoparticle-based carriers, further strengthening its market dominance.
North America Remains a key hub DNA Vaccine price drivers Innovation and Growth.
Supplier Landscape: Supplier Negotiations and Strategies
The supplier landscape in the DNA vaccine market is dynamic and increasingly competitive, featuring a combination of global pharmaceutical giants and specialized biotechnology firms. These suppliers significantly influence factors such as production costs, technological innovation, and delivery efficiency. Established players dominate the market by offering comprehensive DNA vaccine solutions, while smaller, niche suppliers focus on customized formulations and advanced delivery systems.
The DNA vaccine supplier ecosystem spans major biotechnology hubs, encompassing leading organizations and emerging innovators that cater to global and regional demand. With the rising adoption of DNA vaccines across infectious diseases, oncology, and other therapeutic areas, suppliers are scaling up production, embracing cutting-edge manufacturing technologies, and prioritizing sustainability to deliver safe, effective, and affordable vaccines.
- Key Suppliers in the DNA Vaccine Market Include:
- Pfizer Inc.
- Moderna, Inc.
- Inovio Pharmaceuticals, Inc.
- Sanofi S.A.
- Zydus Cadila
- Johnson & Johnson
- Gene One Life Science
- CureVac N.V.
- Takara Bio Inc.
- Aldevron, LLC
Key Developments Procurement Category Significant Development:
Significant Development |
Description |
Market Growth |
The DNA vaccine market is experiencing robust expansion, driven by increasing applications in infectious diseases, oncology, and other therapeutic areas, particularly in developing regions. |
Sustainability Focus |
There is growing emphasis on sustainable production practices, with manufacturers adopting energy-efficient processes and minimizing waste to reduce the environmental impact of vaccine development. |
Product Innovation |
Companies are diversifying their offerings, introducing advanced DNA vaccine formulations, improved delivery systems, and vaccines targeting specific pathogens or diseases. |
Technological Advancements |
Innovations such as next-generation plasmid production, advanced electroporation techniques, and scalable manufacturing platforms are enhancing vaccine efficacy and production efficiency. |
Global Trade Dynamics |
Evolving international trade regulations, supply chain disruptions, and geopolitical factors are influencing the availability of raw materials and the global distribution of DNA vaccines. |
Customization Trends |
There is rising demand for tailored DNA vaccines, such as region-specific vaccines for emerging pathogens and personalized immunotherapies for cancer treatment. |
|
DNA Vaccine Attribute/Metric |
Details |
Market Sizing |
The global DNA vaccines market is projected to reach USD 774.43billion by 2032, growing at a CAGR of approximately 6.3 % from 2024 to 2032. |
DNA Vaccine Technology Adoption Rate |
Around 60% of biotechnology firms and pharmaceutical companies are adopting advanced DNA synthesis and delivery technologies to enhance vaccine efficacy and scalability. |
Top DNA Vaccine Industry Strategies for 2024 |
Key strategies include focusing on scalable manufacturing platforms, expanding vaccine pipelines, improving delivery methods, and ensuring cost-effective production. |
DNA Vaccine Process Automation |
Approximately 45% of vaccine manufacturers have implemented automation in plasmid production, quality testing, and delivery system development to improve efficiency and maintain high standards. |
DNA Vaccine Process Challenges |
Major challenges include addressing regulatory compliance, ensuring raw material availability, managing production scalability, and meeting global demand for rapid vaccine distribution. |
Key Suppliers |
Leading players in the DNA vaccine market include Inovio Pharmaceuticals (USA), Zydus Cadila (India), and Genexine Inc. (South Korea), offering diverse DNA vaccine solutions. |
Key Regions Covered |
Prominent regions for DNA vaccine development include North America, Asia-Pacific, and Europe, with significant advancements in infectious disease and oncology applications. |
Market Drivers and Trends |
Growth is driven by rising investments in vaccine R&D, technological advancements in plasmid design and delivery, increasing government support, and the growing focus on personalized medicine. |










