Summary Overview
Clinical Trials Market Overview:
The global clinical trials market is witnessing consistent growth, driven by the increasing demand for innovative therapies across various therapeutic areas such as oncology, cardiology, neurology, and infectious diseases. The market encompasses various types of trials, including preclinical, phase I-IV, and post-marketing studies. Our analysis offers an in-depth look at procurement trends in clinical trials, focusing on cost management strategies and the utilization of digital tools to streamline procurement and trial processes.
Key future challenges in clinical trials procurement include managing the rising costs of trial conduct, navigating regulatory complexities, and ensuring timely patient recruitment and retention. The application of digital tools and strategic sourcing will be crucial in optimizing the clinical trial supply chain and boosting long-term success. As global demand continues to grow, organizations are leveraging market intelligence to improve trial efficiency and mitigate risks.
The clinical trials market is expected to experience steady growth through 2032, with key projections as follows:
-
Market Size: The global clinical trials market is forecasted to reach USD 106.78 billion by 2032, growing at a CAGR of approximately 7.1% from 2024 to 2032.
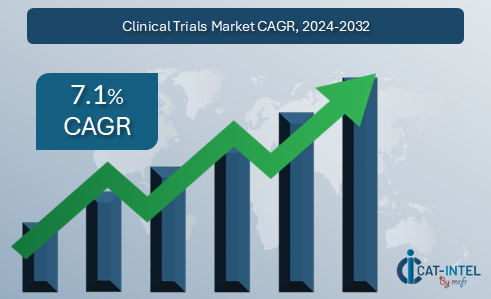
Growth Rate: 7.1%
-
Sector Contributions: The market's expansion is driven by: -
Oncology Trials: Rising demand for cancer treatments, including immunotherapies and personalized medicine, is driving the growth of clinical trials in oncology. -
Cardiology Trials: Ongoing advancements in heart disease treatments, including gene therapies and innovative medical devices, are leading to increased clinical trial activity in cardiology. -
Digital Health: The integration of digital health technologies, including remote patient monitoring, telemedicine, and AI-powered analytics, is transforming trial management and patient engagement. -
Data Integration: Advanced data integration systems are enhancing trial monitoring, reducing errors, and improving decision-making processes. -
Investment in Infrastructure: Pharmaceutical companies are investing in advanced clinical trial management systems (CTMS) and other technologies to enhance trial efficiency and reduce costs. -
Regional Insights: North America remains a dominant player in the clinical trials market due to its robust healthcare infrastructure, high research investment, and established regulatory framework.
Key Trends and Sustainability Outlook:
-
Digitization in Clinical Trials: The increased use of electronic data capture (EDC), electronic health records (EHR), and decentralized trials is improving the efficiency and speed of trial execution. -
Personalized Medicine: Growing interest in precision medicine is driving demand for clinical trials focused on genetic profiling and targeted therapies. -
Sustainability Focus: There is an increasing focus on reducing the environmental impact of clinical trials, including minimizing waste and utilizing sustainable practices in clinical trial management. -
Patient-Centric Trials: Efforts to enhance patient engagement and recruitment are making trials more accessible and efficient, particularly with the rise of virtual trials and direct-to-patient models.
Growth Drivers:
-
Rising Prevalence of Chronic Diseases: An increasing burden of chronic diseases such as cancer, cardiovascular conditions, and diabetes is driving the demand for clinical trials. -
Regulatory Changes: The evolving regulatory landscape and accelerated approval pathways are fuelling the demand for faster and more efficient clinical trials. -
Investment in Research and Development: Growing investments in pharmaceutical and biotechnology R&D are contributing to the expansion of clinical trials. -
Global Expansion: The increasing globalization of clinical trials, particularly in emerging markets, is providing access to diverse patient populations and improving trial outcomes. -
Patient Recruitment and Retention: Efforts to streamline patient recruitment and retention strategies are essential for ensuring timely completion of trials.
Overview of Market Intelligence Services for Clinical Trials:
Recent reports have underscored key challenges such as managing clinical trial costs, ensuring compliance with global regulatory standards, and addressing patient recruitment hurdles. Market intelligence services offer actionable insights into procurement opportunities, enabling organizations to identify cost-saving strategies, improve vendor management, and strengthen the resilience of trial operations. These reports also support organizations in meeting regulatory requirements and maintaining high-quality standards while managing operational costs.
Procurement Intelligence for Clinical Trials: Category Management and Strategic Sourcing:
To remain competitive in the clinical trials market, companies are optimizing procurement strategies by employing spend analysis to track vendor performance and enhance supply chain efficiency. Effective category management and strategic sourcing are crucial for reducing procurement costs and ensuring a steady supply of clinical trial materials and services. By leveraging market intelligence, organizations can refine their procurement approaches, secure optimal terms for trial services, and streamline their trial operations.
Pricing Outlook for Clinical Trials: Spend Analysis
The pricing outlook for clinical trials is anticipated to remain relatively steady, though fluctuations may arise due to several influencing factors. Variations in operational costs, such as patient recruitment, investigator fees, regulatory compliance, and technological investments, can significantly impact price trends. Additionally, the rising demand for faster, more efficient, and digitally integrated clinical trial processes is contributing to price pressures.
Graph shows general upward trend pricing for clinical trials and growing demand. However, there may be fluctuations influenced by economic conditions, technological advancements, and competitive dynamic.
Efforts to enhance operational efficiency, reduce overhead, and incorporate advanced technologies like AI-driven data analysis, real-time monitoring systems, and automated patient monitoring are essential for managing costs. Developing innovative clinical trial management solutions, enhancing patient engagement, and improving trial protocols can help mitigate pricing challenges.
Collaboration with technology providers, investment in digital platforms, and optimizing trial logistics are crucial strategies for controlling costs. Despite these challenges, maintaining a strong focus on patient recruitment, service quality, regulatory compliance, and continuous technological advancements will be key to managing pricing effectively.
Cost Breakdown for Clinical Trials: Total Cost of Ownership (TCO) and Cost-Saving Opportunities
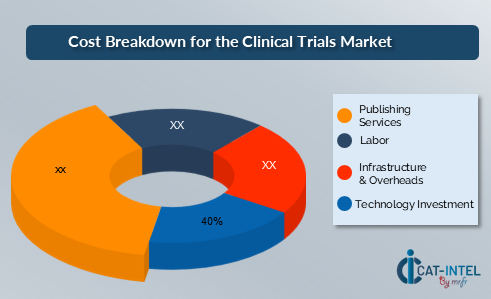
- Technology Investments (40%)
- Description: Technology investments include clinical trial management systems (CTMS), electronic data capture (EDC) tools, AI-based analytics platforms, and remote patient monitoring systems. These technologies are crucial for improving trial efficiency, ensuring accurate data collection, and enhancing patient engagement.
- Trends: Increased adoption of cloud-based solutions, AI, and automation tools to enhance trial data management and speed up the decision-making process. The demand for scalable, secure, and flexible trial platforms is rising as companies aim to optimize their clinical workflows.
- Labor (XX%)
- Publishing Services (XX%)
- Infrastructure & Overheads (XX%)
Cost-Saving Opportunities: Negotiation Levers and Purchasing Negotiation Strategies
In the clinical trials industry, optimizing procurement processes and improving operational efficiencies can lead to substantial cost savings and enhanced trial delivery. Establishing long-term relationships with key service providers, including clinical research organizations (CROs), data management firms, and third-party vendors, can help secure favourable pricing, reduce service fees, and improve overall operational efficiency. Strategic partnerships with technology providers and outsourcing facilities can offer benefits such as better payment terms, scalable solutions, and cost-effective trial management services.
Investing in automation technologies, AI-driven data analysis, and cloud-based trial management systems can reduce operational costs, minimize manual errors, and accelerate trial timelines. Additionally, optimizing the use of digital platforms for patient recruitment and monitoring can further reduce operational and recruitment-related costs. Multi-sourcing strategies, including diversifying trial management partners and leveraging competitive bidding processes, can mitigate risks related to service disruptions and enhance negotiating power.
Supply and Demand Overview for Clinical Trials: Demand-Supply Dynamics and Buyer Intelligence for Effective Supplier Relationship Management (SRM)
The clinical trials services market is experiencing steady growth, fuelled by increasing demand across various therapeutic areas, including oncology, cardiology, and neurology. The balance between supply and demand is influenced by factors such as regulatory changes, technological innovations, and evolving patient expectations.
Demand Factors:
-
Digital Transformation: Growing demand for digital and automated trial solutions as organizations seek faster, more accurate, and cost-effective methods to manage trials and gather data. -
Patient Expectations: Rising consumer demand for personalized treatments and faster trial recruitment processes is driving service providers to adopt innovative solutions that meet these expectations. -
Regulatory Compliance: Increasing regulatory requirements in the pharmaceutical and biotechnology sectors are creating demand for specialized trial management solutions that ensure compliance with local and international standards. -
Outsourcing Trends: As companies look to reduce overhead costs, there is a rising trend toward outsourcing clinical trial management to third-party providers offering scalable, cost-effective services.
Supply Factors:
-
Technology Advancements: Innovations in AI, machine learning, and cloud computing are enabling trial managers to improve efficiency, reduce errors, and enhance patient monitoring and data collection. -
Skilled Workforce Availability: The availability of experienced clinical researchers, data analysts, and medical experts is essential for delivering high-quality trial services and ensuring compliance with regulatory standards. -
Data Security and Compliance: Clinical trials involve sensitive patient data, making robust security measures and compliance with privacy regulations vital for service providers. -
Vendor Competition: Intense competition among clinical trial service providers is driving improvements in service quality, trial timelines, and competitive pricing, benefiting trial sponsors and organizations.
Regional Demand-Supply Outlook: Clinical Trials
The Image shows growing demand for clinical trials in both North America and Asia Pacific with potential price increases and increased Competition.
North America: Dominance in Clinical Trials Services
North America, particularly the United States and Canada, plays a dominant role in the global clinical trials market, driven by several key factors:
-
Advanced Technological Capabilities: North America is home to leading clinical trial technology providers offering cutting-edge solutions in digital patient monitoring, AI-driven data analysis, and decentralized trial models. -
Strong Regulatory Environment: The region benefits from a well-established regulatory framework that demands high standards in clinical trial operations, making it a hub for specialized, compliant solutions. -
Skilled Labor and Innovation: North America boasts a highly skilled labor force and a culture of innovation, enabling companies to implement the latest technologies and maintain high-quality trial delivery. -
Market Size and Growth: The North American market is the largest consumer of clinical trials services, driven by the demand from pharmaceutical, biotechnology, and healthcare sectors. This region also serves as a significant exporter of clinical trial solutions and technologies to other parts of the world, including Europe and Asia.
Supplier Landscape: Supplier Negotiations and Strategies
The supplier landscape in the clinical trials market is highly competitive, consisting of both large global service providers and smaller, specialized firms catering to specific therapeutic areas or trial phases. These suppliers play a crucial role in influencing market dynamics such as pricing, service quality, and operational efficiency. While established global players dominate the market, smaller, niche providers focus on specialized services, such as patient recruitment, data management, and regulatory compliance, for specific sectors like oncology, cardiology, and neurology.
The clinical trials supplier landscape is diverse, particularly in regions with high demand for trial services, with both well-established firms and emerging players addressing both global and local market needs. As demand for more efficient, cost-effective, and patient-centric clinical trial solutions increases, suppliers are emphasizing technological advancements, service innovation, and strategic partnerships to strengthen their market position. Additionally, a focus on regulatory compliance, data security, and adherence to industry standards is driving suppliers to continuously improve their offerings to meet the evolving needs of sponsors, research organizations, and patients.
Key Suppliers in the Clinical Trials Market Include:
- Parexel International
- Celeron
- Covance (LabCorp Drug Development)
- IQVIA
- Medidata Solutions
- Charles River Laboratories
- Syneos Health
- PRA Health Sciences
- CROMSOURCE
- KCR
Key Developments Procurement Category Significant Development:
Significant Development |
Description |
Market Growth |
The global clinical trials market is experiencing significant growth, driven by the increasing demand for new treatments, rising healthcare needs, and technological advancements. |
Sustainable Practices |
Growing emphasis on sustainable trial practices, including ethical patient recruitment, data privacy compliance, and environmentally responsible trial operations. |
Service Diversification |
Expansion of clinical trial services, incorporating digital, decentralized, and AI-driven solutions to cater to diverse therapeutic areas and patient needs. |
Technological Innovations |
Adoption of cutting-edge technologies, such as AI for data analysis, automation in trial management, and cloud-based platforms, improving trial efficiency, accuracy, and monitoring. |
E-commerce Expansion |
The rise of digital platforms for clinical trial management, improving access for sponsors, sites, and patients, and streamlining the trial process for better outcomes. |
Focus on Efficiency |
Growing demand for faster, more efficient trial processes, such as real-time patient monitoring, automated data entry, and accelerated recruitment, to shorten trial timelines. |
Clinical Trials Service Attribute/Metric |
Details |
Clinical Trials Market Sizing |
The global clinical trials market is forecasted to reach USD 106.78 billion by 2032, growing at a CAGR of approximately 7.1% from 2024 to 2032. |
Technology Adoption Rate in Clinical Trials |
Approximately 35% of clinical trial organizations are adopting advanced technologies, such as AI for data analysis, decentralized trials, and cloud-based management platforms to improve efficiency and accuracy. |
Top Clinical Trials Strategies for 2024 |
Focus on accelerating digital transformation, improving patient recruitment through digital platforms, enhancing data security, and implementing decentralized trial models for faster and more accurate results. |
Clinical Trials Automation |
25% of clinical trial organizations have automated key processes such as patient recruitment, data collection, and reporting to improve efficiency and reduce timelines. |
Clinical Trials Challenges |
Major challenges include managing complex regulatory requirements, ensuring patient data privacy, addressing recruitment challenges, and adapting to evolving clinical trial needs. |
Key Suppliers |
Leading suppliers in the clinical trials services market include Parexel, IQVIA, Covance, Syneos Health, and Charles River Laboratories, offering comprehensive solutions across various therapeutic areas. |
Key Regions Covered |
North America, Europe, and Asia-Pacific dominate the clinical trials services market, with significant demand driven by the pharmaceutical, biotechnology, and healthcare industries. |
Market Drivers and Trends |
Growth is driven by increasing demand for decentralized trials, advancements in AI and data analytics for faster decision-making, and rising pressure for compliance with stringent regulatory standards in healthcare and pharmaceuticals. |










