Summary Overview
Clinical Trial Supply Market Overview:
The global clinical trial supply market is experiencing steady growth, driven by increasing demand for efficient and reliable supply chains across the pharmaceutical and healthcare sectors. This market encompasses the management, distribution, and logistics of clinical trial materials, including drugs, medical devices, and biologics, which are critical for successful clinical trials. Our report provides an in-depth analysis of procurement trends, focusing on cost optimization strategies and the use of digital tools to streamline procurement and supply chain processes.
Key future challenges in procurement include managing fluctuating drug prices, ensuring timely delivery of materials, and addressing regulatory compliance. Digital procurement tools and strategic sourcing are key to optimizing clinical trial supply chains and enhancing long-term competitiveness. As the demand for clinical trials grows, companies are increasingly relying on market intelligence to improve efficiency and mitigate risks.
The clinical trial supply market is expected to maintain steady growth through 2032, with key highlights including:
-
Market Size: The global clinical trial supply market is projected to reach USD 8.58 billion by 2032, growing at a CAGR of approximately 7.63 % from 2024 to 2032.
Growth Rate: 7.63%
-
Sector Contributions: Growth in the market is driven by: -
Pharmaceutical Industry Growth: The increasing number of clinical trials for drug development, especially in oncology, immunology, and gene therapies. -
Regulatory and Compliance Needs: The rise in regulatory requirements and the need for precision in clinical trial supplies to ensure patient safety and accurate results. -
Technological Transformation and Innovations: Advancements in supply chain management, such as the use of AI and machine learning for inventory tracking, temperature monitoring, and logistics optimization, are improving efficiency and reducing the risk of delays. -
Investment Initiatives: Companies are investing in advanced distribution technologies, such as temperature-controlled packaging and real-time monitoring systems, to ensure the integrity of clinical trial supplies and reduce costs. -
Regional Insights: North America remains a significant contributor to the clinical trial supply market due to its robust healthcare infrastructure and the large number of ongoing clinical trials.
Key Trends and Sustainability Outlook:
-
Enhanced Digital Integration: The use of automated systems in clinical trial supply management is improving accuracy, reducing errors, and optimizing workflows. -
Advanced Packaging Materials: Innovative packaging solutions, such as tamper-proof and temperature-sensitive packaging, are ensuring the safety and integrity of trial supplies. -
Focus on Sustainability: There is a growing emphasis on reducing the environmental impact of clinical trial logistics and packaging by adopting eco-friendly materials and processes. -
Customization Trends: There is an increasing demand for specialized trial supplies tailored to the specific requirements of each clinical study. -
Data-Driven Supply Chain Management: Leveraging big data and analytics to predict demand and optimize the logistics and distribution of clinical trial materials.
Growth Drivers:
-
Expanding Drug Development: Ongoing investment in clinical research for new drugs, particularly in areas such as oncology and neurology, is driving demand for clinical trial supplies. -
Regulatory Compliance: The increasing complexity of regulatory standards is necessitating advanced logistics and supply chain management solutions. -
Technological Advancements: The adoption of digital tools, such as AI and blockchain, in supply chain management is improving efficiency and transparency. -
Customization Needs: The rise in personalized medicine and targeted therapies is increasing the demand for customized clinical trial supplies. -
Global Clinical Trials: The expansion of clinical trials into emerging markets is driving the need for effective and scalable supply chain solutions.
Overview of Market Intelligence Services for Clinical Trial Supply:
Recent analyses have highlighted key challenges such as fluctuations in material costs and the complexities involved in managing global supply chains. Market intelligence reports provide actionable insights into procurement opportunities, helping companies identify cost-saving measures, optimize vendor management, and enhance supply chain resilience. These reports also help organizations navigate regulatory requirements and ensure high-quality standards while effectively managing costs.
Procurement Intelligence for Clinical Trial Supply: Category Management and Strategic Sourcing:
To stay competitive in the clinical trial supply market, companies are optimizing procurement processes through spend analysis, vendor tracking, and improving supply chain efficiency using market intelligence. Effective category management and strategic sourcing are essential for reducing procurement costs and ensuring a reliable supply of clinical trial materials. By leveraging actionable market intelligence, businesses can enhance their procurement strategies and secure the best possible terms for their clinical trial supply needs.
Pricing Outlook for Clinical Trial Supply (CTS) Services: Spend Analysis
The pricing outlook for clinical trial supply (CTS) services is expected to remain relatively stable, although fluctuations may occur due to several influencing factors. Variations in operational costs, including raw material prices, logistics, regulatory requirements, and staffing, can significantly impact price trends. Additionally, the growing demand for more efficient, timely, and regulated clinical trial supplies is contributing to price pressures.
Graph shows general upward trend pricing for clinical trial supply and growing demand. However, there may be fluctuations influenced by economic conditions, technological advancements, and competitive dynamic.
Efforts to improve operational efficiency, reduce overhead, and integrate advanced technologies like temperature-sensitive packaging, automated inventory systems, and real-time supply chain monitoring are crucial for managing costs. The development of innovative packaging, supply management solutions, and improved customer service capabilities can help mitigate pricing challenges.
Collaborating with technology providers, investing in digital supply chain platforms, and optimizing distribution logistics are essential strategies for controlling costs. Despite these challenges, maintaining a strong focus on regulatory compliance, service quality, and continuous innovation will be key to managing pricing effectively.
Cost Breakdown for Clinical Trial Supply Services: Total Cost of Ownership (TCO) and Cost-Saving Opportunities.
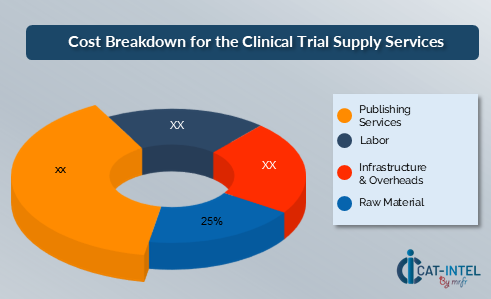
- Raw Materials (25%)
- Description: Raw materials for clinical trial supplies include the cost of drugs, biologicals, medical devices, and packaging materials required for the trial. These costs are often influenced by market fluctuations and regulatory requirements.
- Trends: Rising prices of raw materials, particularly for specialized and customized supplies, are affecting overall costs. The demand for sustainable materials in packaging is also impacting raw material costs.
- Labor (XX%)
- Publishing Services (XX%)
- Infrastructure & Overheads (XX%)
Cost-Saving Opportunities: Negotiation Levers and Purchasing Negotiation Strategies
In the Clinical Trial Supply (CTS) services industry, optimizing procurement processes and enhancing operational efficiencies can lead to substantial cost savings and improved service delivery. Establishing long-term partnerships with key suppliers, logistics providers, and regulatory service firms can help secure favourable pricing, reduce service fees, and improve overall operational performance. Strategic collaborations with drug manufacturers, packaging vendors, and third-party logistics providers can offer benefits such as better payment terms, scalable solutions, and cost-effective supply management services.
Investing in advanced supply chain technologies, real-time tracking systems, and automated inventory management can lower operational costs, minimize errors, and streamline the procurement process. Additionally, incorporating energy-efficient technologies in storage and transportation, as well as optimizing the use of distribution resources, can further reduce infrastructure-related costs. Adopting multi-sourcing strategies, including diversifying suppliers and leveraging competitive bidding processes, can mitigate risks related to supply chain disruptions and enhance negotiation power.
Supply and Demand Overview for Clinical Trial Supply (CTS): Demand-Supply Dynamics and Buyer Intelligence for Effective Supplier Relationship Management (SRM)
The clinical trial supply market is experiencing steady growth, driven by the increasing demand for efficient and reliable supply chain solutions in the pharmaceutical and healthcare industries. The balance between supply and demand is influenced by several factors, including regulatory requirements, technological advancements, and evolving customer expectations.
Demand Factors:
-
Digital Transformation: Growing demand for digital and automated solutions in clinical trial supply management as organizations seek faster, more accurate, and cost-effective methods for trial supply tracking and logistics. -
Regulatory Compliance: Increasing regulatory requirements in the pharmaceutical industry are driving the demand for specialized clinical trial supply services that ensure compliance with stringent safety standards. -
Clinical Trial Growth: The rise in global clinical trials, particularly for new drug development, is creating greater demand for clinical trial supplies, including customized packaging and temperature-sensitive materials. -
Outsourcing Trends: As pharmaceutical companies look to reduce costs and improve operational efficiency, there is a growing trend towards outsourcing clinical trial supply management to specialized third-party providers offering scalable and cost-effective services.
Supply Factors:
-
Technology Advancements: Innovations in AI, machine learning, and cloud-based supply chain solutions are enabling CTS providers to improve efficiency, reduce errors, and enhance overall service quality. -
Skilled Workforce Availability: The availability of experienced professionals in clinical trial supply chain management, logistics, and regulatory affairs is critical for ensuring high-quality services and meeting industry standards. -
Data Security and Compliance: As clinical trial supply services involve sensitive and regulated materials, ensuring robust data security measures and compliance with privacy regulations is essential for service providers. -
Vendor Competition: Intense competition among CTS service providers is driving improvements in service quality, faster delivery times, and competitive pricing, benefiting buyers by providing more cost-effective and efficient supply chain solutions.
Regional Demand-Supply Outlook: Clinical Trial Supply (CTS)
The Image shows growing demand for clinical trial supply in both North America and Asia Pacific with potential price increases and increased Competition.
North America: Dominance in Clinical Trial Supply Services
North America, particularly the United States and Canada, plays a dominant role in the global clinical trial supply (CTS) market, driven by several key factors:
-
Advanced Technological Capabilities: North America is home to some of the world’s leading clinical trial supply technology providers, offering cutting-edge solutions in real-time tracking, automated inventory management, and temperature-sensitive logistics. -
Strong Regulatory Environment: The region has a well-established regulatory framework that ensures compliance with stringent standards in clinical trial supply chain management, making it a hub for specialized, compliant solutions. -
Skilled Labor and Innovation: North America benefits from a highly skilled labor force with expertise in clinical trial logistics, regulatory affairs, and supply chain management, allowing companies to implement the latest technologies and maintain high standards of service delivery. -
Market Size and Growth: The North American market is the largest consumer of clinical trial supply services, with significant demand driven by pharmaceutical companies, biotechnology firms, and healthcare organizations.
Supplier Landscape: Supplier Negotiations and Strategies
The supplier landscape in the Clinical Trial Supply (CTS) services market is highly competitive, consisting of both large, global service providers and smaller, specialized firms that focus on specific sectors such as pharmaceuticals, biotechnology, and healthcare. These suppliers play a crucial role in shaping market dynamics, including pricing, service quality, and operational efficiency. The market is competitive, with major players leading the industry, while smaller, niche providers specialize in delivering tailored CTS solutions for specific therapeutic areas or regions.
In regions with high demand for clinical trial supply services, the supplier landscape is diverse, with established firms and emerging players addressing both global and localized needs. As the demand for more efficient, cost-effective, and compliant CTS solutions grows, suppliers are prioritizing technological advancements, service innovation, and strategic partnerships to strengthen their market position. Additionally, a strong focus on regulatory compliance, data security, and adherence to industry standards drives suppliers to continuously enhance their offerings to meet evolving customer expectations.
Key Suppliers in the Clinical Trial Supply (CTS) Services Market Include:
- Lonza Group
- Wuxi App Tec
- Catalent
- Kuehne + Nagel
- Parexel
- Alcami Corporation
- Celeron
- Pall Corporation
- Worldwide Clinical Trials
- Med pace
Key Developments Procurement Category Significant Development:
Significant Development |
Description |
Market Growth |
The global Clinical Trial Supply (CTS) market is experiencing growth, driven by increasing demand from the pharmaceutical, biotechnology, and healthcare sectors. |
Sustainable Practices |
Growing emphasis on ethical sourcing, environmentally responsible packaging, and adherence to sustainability standards in the supply of clinical trial materials. |
Service Diversification |
Expansion of CTS services, including temperature-controlled logistics, customized packaging, and specialized supply chain management to meet the needs of various trials. |
Technological Innovations |
Adoption of advanced technologies, such as real-time tracking systems, automated inventory management, and cloud-based platforms, enhancing efficiency and service. |
E-commerce Expansion |
Growth in digital platforms for clinical trial supply management, improving access for pharmaceutical companies and streamlining processes for global trials. |
Focus on Efficiency |
Increasing demand for faster, more efficient supply chains in clinical trials, utilizing automated systems and real-time monitoring to improve trial timelines and accuracy. |
Clinical Trial Supply (CTS) Service Attribute/Metric |
Details |
Clinical Trial Supply Market Sizing |
The global clinical trial supply market is projected to reach USD 8.58 billion by 2032, growing at a CAGR of approximately 7.63 % from 2024 to 2032. |
Technology CTS Service Adoption Rate |
Around 45% of companies in the CTS industry are adopting advanced technologies, such as real-time tracking systems, automated inventory management, and cloud-based platforms, to improve operational efficiency and accuracy. |
Top CTS Strategies for 2024 |
Focus on enhancing supply chain visibility, integrating real-time monitoring systems, improving cold chain management, and increasing automation in logistics and inventory management. |
CTS Automation |
Approximately 35% of CTS companies have automated key processes such as supply chain tracking, packaging, and inventory management, to improve efficiency and reduce delays. |
CTS Challenges |
Major challenges include managing global supply chains, ensuring regulatory compliance, maintaining product quality, and meeting the diverse needs of clinical trials. |
Key Suppliers |
Leading suppliers in the CTS market include Lonza, Wuxi AppTec, Catalent, Kuehne + Nagel, and Parexel, providing end-to-end solutions for clinical trials across various therapeutic areas. |
Key Regions Covered |
North America, Europe, and Asia-Pacific dominate the CTS market, with significant demand driven by pharmaceutical companies, biotech firms, and healthcare providers. |
Market Drivers and Trends |
Growth is driven by increasing demand for efficient and reliable clinical trial supply chains, technological innovations in tracking and automation, and the rising focus on regulatory compliance and patient-centric trial models. |










