Summary Overview
Cell Therapy Market Overview
The Cell Therapy Market is experiencing significant growth due to advancements in regenerative medicine, cancer treatment, and chronic disease management. This sector includes innovations such as CAR-T cell therapies, mesenchymal stem cells, and hematopoietic stem cells. These therapies have applications in oncology, musculoskeletal conditions, and cardiovascular treatments.
The market is expected to expand significantly by 2032, fuelled by rising product approvals, the growing prevalence of chronic diseases, and increasing investments in research. The adoption of novel therapies for cancer and other critical conditions is also a key driver of growth.
Market Size and Growth Rate
-
Market Size: The cell therapy market is projected to reach USD 16 billion by 2032, growing at a CAGR of 24.4% from 2024 to 2032.
Growth rate: 24.4%
Sector Contributions
-
Oncology: Advances in cell therapies, particularly CAR-T treatments, are driving significant growth in the oncology segment, especially for blood cancers. -
Musculoskeletal and Cardiovascular: Research into stem cell applications for musculoskeletal and heart diseases is contributing to the sector's expansion. -
Regenerative Medicine: The use of stem cells in regenerative medicine is rapidly increasing, enabling new treatment options for various conditions.
Technological Transformations and Innovations
-
CAR-T Therapies: Gene-modified T-cell therapies, such as CAR-T therapies for cancer, are making substantial progress, exemplified by FDA-approved therapies like Breyanzi and Yes carta. -
Manufacturing Advancements: Enhanced manufacturing processes, such as reducing the production time for CAR-T therapies, are improving supply chain efficiency and accessibility.
Funding Initiatives
-
Increased Investment: Funding from both government and private sectors is accelerating research, particularly for cancer and chronic disease treatments, pushing forward the development of new cell therapy options.
Regional Insights
-
North America: The U.S. is leading the cell therapy market due to favourable regulatory approvals, heavy investments, and significant clinical trials. -
Europe and Asia: Cell therapy adoption is growing rapidly in Europe and Asia, driven by increased investments in clinical trials and growing demand for effective therapies.
Key Trends and Sustainability Outlook
-
Sustainability: Cell therapy manufacturers are focusing on improving production efficiency and scalability to make therapies more accessible and cost-effective. -
Health Trends: The increasing incidence of chronic diseases is propelling the demand for advanced cell therapies.
Growth Drivers
-
Chronic Disease Prevalence: The rising global burden of chronic diseases, particularly cancer, cardiovascular diseases, and musculoskeletal disorders, is a key factor driving the demand for advanced therapies. -
Product Approvals: The steady stream of FDA approvals for cell therapies is contributing to the growth of the market.
Overview of Market Intelligence Services for the Cell Therapy Market
Given the high costs and complex production processes associated with cell therapies, market intelligence services provide valuable insights into pricing trends, procurement strategies, and risk management. These insights help stakeholders make informed purchasing decisions, manage risks, and optimize their supply chains. Strategic sourcing and vendor management are essential to maintaining the availability of high-quality therapies while managing costs in a competitive and evolving market.
Procurement Intelligence for Cell Therapy Market: Category Management and Strategic Sourcing
In the Cell Therapy Market, procurement strategies are being optimized to address the complexities of sourcing biologics, raw materials, and specialized equipment necessary for manufacturing cell therapies. Companies are increasingly adopting spend analysis solutions to better understand vendor expenditures and improve cost-efficiency. This approach allows for the identification of potential cost-saving opportunities through strategic sourcing and vendor management.
Pricing Outlook for Cell Therapy Market: Spend Analysis
The Cell Therapy Market is navigating a dynamic pricing landscape, influenced by various factors such as technological advancements, regulatory approvals, and the increasing demand for innovative therapies. The pricing environment for cell therapies, particularly for high-cost treatments like CAR-T therapies, is shaped by several key trends and challenges, leading to fluctuations in treatment cost.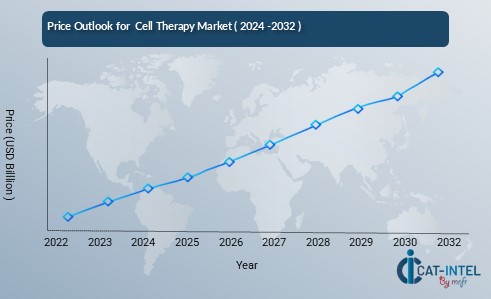
Our advanced analysis indicates several factors driving the pricing trends in the cell therapy market:
-
Rising Production Costs: The complex and labour-intensive production processes for cell therapies, including patient-specific treatments, significantly contribute to high costs. The need for specialized facilities, skilled labour, and quality control drives up expenses. -
Surge in Demand: The increasing global prevalence of chronic diseases, including cancer, and the growing adoption of innovative therapies, particularly CAR-T cell therapies for oncology, are pushing demand and influencing pricing. -
Regulatory Impact: The regulatory requirements for approval, safety testing, and compliance add significant costs to the development and commercialization of cell therapies. As more therapies receive approval, pricing is expected to stabilize but remain high due to the complexities involved. -
Technological Advancements: Ongoing research and the introduction of newer, more efficient manufacturing technologies are expected to gradually lower production costs over time, though it will take years for these savings to be reflected in the market price. -
Global Market Dynamics: The competitive pricing landscape is affected by increased production capabilities in North America, Europe, and Asia, especially in regions investing heavily in cell therapy infrastructure. Additionally, the growing international market for cell therapies, particularly in emerging markets, adds pressure on pricing.
Cost Breakdown for Cell Therapies Market: Cost Saving Opportunities
- Raw Materials (45%)
-
Description: This includes the cost of acquiring stem cells, genetic material, and biological reagents required for cell therapy production. These materials are typically sourced from specialized suppliers and are critical for maintaining the quality of cell therapies like CAR-T and stem cell therapies. -
Trends: Raw material costs can be volatile due to factors like supplier shortages, stringent regulatory requirements, and the need for highly specialized products. The ongoing evolution of sourcing technologies and biological advancements may offer cost reduction opportunities in the future.
- Labor (XX%)
- Manufacturing & Processing (XX%)
- Regulatory & Compliance (XX%)
Cost saving opportunity: Negotiation Lever and Purchasing Negotiation Strategies
In the cell therapy market, cost savings can be achieved through strategic procurement tactics such as collaborative purchasing for bulk discounts on raw materials, leveraging automation to reduce labour costs, and negotiating long-term contracts with suppliers to stabilize pricing. Outsourcing manufacturing processes and adopting sustainable practices, like energy-efficient facilities, further reduce operational expenses. Additionally, optimizing logistics and negotiating favourable terms with transportation providers can lower shipping costs. These strategies, combined with efficient clinical trial and regulatory processes, can significantly enhance cost efficiency and profitability in the cell therapy sector.
Supply and Demand Overview of the Cell Therapy Market: Demand-Supply Dynamics and Buyer Intelligence for Effective Supplier Relationship Management (SRM)
The cell therapy market is experiencing significant growth, driven by advancements in regenerative medicine, increasing healthcare demands, and innovations in cellular treatments. The demand for cell therapies, including CAR-T, stem cell treatments, and gene therapies, is strong in oncology, autoimmune diseases, and regenerative medicine, supported by expanding clinical trials, regulatory approvals, and a growing global healthcare market.
Demand Factors:
-
Medical Advancements: The continuous innovation in regenerative medicine and cell-based therapies is driving demand for advanced treatments in oncology, autoimmune disorders, and chronic diseases. -
Rising Healthcare Needs: Increasing global healthcare demands, particularly in aging populations, are fuelling the need for novel therapies, including cell and gene therapies, to address unmet medical needs. -
Personalized Medicine: The shift towards personalized medicine, where treatments are tailored to individual patients, is significantly boosting the demand for cell therapies, especially in cancer treatments. -
Global Healthcare Expansion: As healthcare systems in emerging economies improve, the demand for advanced therapies, including stem cells and gene therapies, is growing, expanding the global market.
Supply Factors:
-
Specialized Manufacturing: The complexity of manufacturing cell therapies requires advanced technologies and specialized facilities, which can limit supply and impact scalability. -
Regulatory Approvals: Regulatory hurdles and stringent approvals are a key factor influencing the pace of supply, as therapies must meet rigorous standards before they are commercialized. -
Cell Sourcing: Sourcing high-quality stem cells, genetic material, and biological reagents remains a critical factor influencing supply availability, with challenges in consistent sourcing from reliable suppliers. -
Supply Chain and Logistics: The global distribution of cell therapies is often hindered by specialized logistics and transportation requirements, such as maintaining temperature-sensitive conditions, influencing supply stability.
Regional Demand-Supply Outlook: Cell Therapy Market
The image shows growing demand for cell therapies in North America, Europe, and Asia, with increasing regulatory approvals, advancing research, and rising healthcare needs driving competition.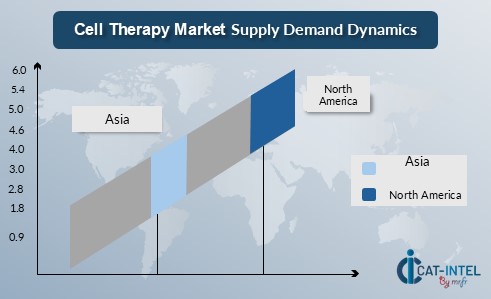
North America: A Key Player in the Cell Therapy Market
North America, particularly the U.S., is a central hub for the development and commercialization of cell therapies, characterized by:
-
Leading Producers: The U.S. is home to several leading companies and research institutions that are pioneering cell therapy technologies, including CAR-T cell therapies, stem cells, and gene therapies, solidifying its position as a global leader in the market. -
Strong Regulatory Support: The U.S. Food and Drug Administration (FDA) has approved several groundbreaking cell therapies, providing a robust regulatory framework that supports market growth and ensures the safety and efficacy of treatments. -
Investment in Research: Heavy investments in R&D are accelerating the development of novel cell therapies, with significant funding from both private and public sectors to advance clinical trials and bring new therapies to market. -
Healthcare Infrastructure: The U.S. benefits from advanced healthcare infrastructure, which facilitates the delivery and distribution of complex cell-based therapies, ensuring their accessibility to a broad patient population. -
Consumer Trends: The rising demand for personalized medicine and the shift toward innovative treatments, particularly in oncology and chronic diseases, are key drivers of growth in the North American cell therapy market.
North America remains a key hub Cell therapies market and its growth
Supplier Landscape: Supplier Negotiations and Strategies for the Cell Therapy Market
The cell therapy market is characterized by a diverse and evolving supplier landscape, with key players providing critical raw materials, equipment, and services essential for the research, development, and production of cell therapies. These suppliers support the complex and regulated process of developing cell-based therapies, such as stem cell therapies, CAR-T therapies, and gene therapies.
Supplier relationships are crucial for the success of cell therapy companies, as high-quality raw materials, advanced equipment, and specialized services are needed to ensure the safety, efficacy, and scalability of these therapies. Collaboration between suppliers and manufacturers focuses on innovation, regulatory compliance, and cost optimization in the production of cell therapies.
Some of the key suppliers in the cell therapy market include:
- Lonza Group
- Miltenyi Biotec
- GE Healthcare Life Sciences
- Thermo Fisher Scientific
- Stemcell Technologies
- Becton Dickinson (BD)
- Cytiva
- AbbVie
- WuXi App Tec
- Sartorius

Key Development: Procurement Category Significant Developments
Category |
Significant Development |
Impact |
Raw Materials |
Growing availability of advanced biomaterials for cell therapy (e.g., culture media, growth factors). |
Increased availability of high-quality raw materials accelerates the development and production of therapies. |
Manufacturing Equipment |
Advancements in automated cell processing technologies (e.g., bioreactors, cell separation technologies). |
Enhanced scalability, precision, and consistency in production. |
Supply Chain Logistics |
Improvements in cold chain logistics and transportation for sensitive cell-based products. |
Ensures the safe and timely delivery of cell therapies, improving patient access to treatments. |
Contract Manufacturing |
Rise in outsourcing to Contract Development and Manufacturing Organizations (CDMOs) for cell therapy production. |
Reduced capital expenditure and access to specialized expertise, accelerating time-to-market. |
Regulatory Compliance |
Stringent regulatory guidelines and harmonization efforts across regions (e.g., FDA, EMA). |
Improved product safety and regulatory adherence, enabling faster approval of new therapies. |
Procurement Attribute/Metric |
Details |
Market Sizing |
The global cell therapy market is projected to grow 16 billion by 2032, with a CAGR of 24.4% during the forecast period. |
Adoption of Cell-Based Therapies |
Increasing adoption of cell therapies for a range of conditions, including cancer, autoimmune diseases, and regenerative medicine, is driving market growth. |
Top Strategies for 2024 |
Focus on increasing production capacity, streamlining regulatory pathways, enhancing cold chain logistics, and advancing cell processing technologies. |
Automation in Cell Therapy Production |
Over 35% of cell therapy manufacturers are adopting automated cell culture systems and closed-loop systems for cell expansion and harvest. |
Procurement Challenges |
Key challenges include the complexity of sourcing high-quality raw materials, managing regulatory compliance, and ensuring the availability of highly specialized manufacturing equipment. |
Key Suppliers |
Major players include Lonza Group, Catalent, WuXi AppTec, and Charles River Laboratories, focusing on providing contract development and manufacturing services for cell therapies. |
Key Regions Covered |
Key markets include North America, Europe, and Asia-Pacific, with strong demand in the U.S., Germany, Japan, and China. |
Market Drivers and Trends |
Growth is driven by advancements in gene and cell therapies, increasing investment in regenerative medicine, and rising demand for personalized medicine. |










