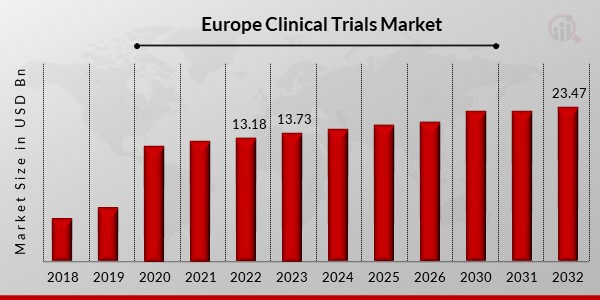
Europe Clinical Trials Market Overview
Europe clinical trials market size was valued at USD 13.18 Billion in 2022 And is projected to grow from USD 13.73 Billion in 2023 to USD 23.47 Billion by 2032, exhibiting a compound annual growth rate (CAGR) of 7.94% during the forecast period (2024 - 2032).
The Europe clinical trials market is emerging due to the increasing number of clinical trials and strong healthcare infrastructure. Additionally, the digital transformation in clinical trials will provide growth opportunities for the market in the future. However, the shortage of skilled and experienced professionals might hamper the market's growth in the forecast period.
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Europe Clinical Trials Market Trends
- Increasing number of clinical trials
In recent years, clinical trials of various drugs and procedures in the European market have increased significantly due to the supportive regulatory environment. Europe has well-established regulatory bodies such as the European Medicines Agency (EMA) and national regulatory agencies that ensure the safety and efficacy of clinical trials. The introduction of the EU Clinical Trials Regulation has streamlined the approval process and harmonized regulations across European countries, making it easier for sponsors to initiate clinical trials. According to the EMA, there has been a consistent increase in the number of clinical trial applications submitted, reflecting the growing interest in conducting trials in Europe. Moreover, the region has a strong network of research institutions, academic centers, and hospitals with state-of-the-art facilities. These facilities provide the necessary infrastructure for conducting clinical trials, including specialized equipment, laboratories, and skilled healthcare professionals.
Furthermore, there is a growing trend towards collaborative networks and partnerships in the European clinical trials market. Collaborative networks, such as the European Clinical Research Infrastructure Network (ECRIN), facilitate the coordination of multinational trials and provide a platform for knowledge exchange among researchers, sponsors, and regulatory authorities. This trend promotes efficiency in trial design, participant recruitment, and data sharing, leading to an increase in the number of clinical trials conducted in Europe
Europe Clinical Trials Market Segment Insights
Europe Clinical Trials market Phase Insights
The Europe Clinical Trials market, based on phase, includes, phase I, Phase II, Phase III, Phase IV. Phase III held the largest market share and phase I is the fastest growing market in the forecast period 2023-2032.
The primary objective of Phase I trials is to establish a safe and tolerable dosage range for subsequent phases. These studies help researchers determine the Maximum Tolerated Dose (MTD) and explore dose escalation strategies. Moreover, Phase I trials provide crucial insights into the drug's behavior within the body, its absorption, distribution, metabolism, and excretion. Phase III trials also continue to assess the safety profile of the investigational treatment in a larger population. Adverse events and side effects are closely monitored, allowing for a comprehensive understanding of the treatment's risk-benefit profile. Phase III trials may also explore subgroup analyses to identify specific patient populations that may benefit the most from the treatment.
Phase IV trials involve the observation and evaluation of patients who are prescribed the approved treatment as part of their routine clinical care. These trials aim to assess the treatment's performance in real-world settings, outside the controlled environment of earlier phases. They provide insights into the treatment's long-term safety profile, potential rare adverse events, drug interactions, and its effectiveness in broader patient populations.
Figure 2: Europe Clinical Trials Market, by Phase, 2023 & 2032 (USD Billion)
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Europe Clinical Trials Study Design Insights
The Europe clinical trials market segmentation, based study design, includes observational, interventional, and expanded access. The Interventional study design held the held the largest market in 2022 and expanded access study design is fastest growing CAGR from 2023 to 2032.
Interventional studies play a crucial role in advancing medical knowledge and improving patient care. They provide valuable data on the safety and effectiveness of investigational treatments, support regulatory approvals, and guide evidence-based clinical practice. The results from well-designed interventional trials inform treatment guidelines, influence clinical decision-making, and contribute to the development of new therapeutic options.
Expanded access programs provide a lifeline for patients who have exhausted all other treatment options and are willing to assume potential risks associated with experimental therapies. These programs also offer valuable insights into the real-world use of investigational treatments, providing additional data on safety, tolerability, and potential effectiveness.
Europe Clinical Trials Market Application Insights
The Europe clinical trials market segmentation, based on application, pharmaceutical, medical devices, nutrition, and others. The pharmaceutical segment held the largest market in 2022. The medical devices segment is expected to register the highest CAGR 2023 to 2032.
Clinical trials conducted in Europe provide an essential pathway for pharmaceutical companies to bring new treatments to the market. These trials involve rigorous testing of experimental drugs or therapies in diverse patient populations across multiple countries, ensuring that the findings are representative of the European population.
Clinical trials for medical devices in Europe are guided by regulatory requirements, including conformity assessment procedures and adherence to relevant standards. Notified bodies, appointed by regulatory authorities, play a crucial role in the evaluation and certification of medical devices, ensuring compliance with safety and performance regulations.
The outcomes of clinical trials in nutrition are vital for informing evidence-based dietary guidelines, nutritional recommendations, and public health policies. Positive trial results contribute to the identification of dietary patterns or specific nutrients that promote health and prevent diseases.
Europe Clinical Trials Market Service Type Insights
The Europe Clinical Trials market has been segmented, based on service type into protocol designing, site identification, patient recruitment, laboratory services, bioanalytical testing services, clinical trial data management services, and others. The protocol designing held the largest market in 2022. The clinical trial data management services segment is expected to register the highest CAGR from 2024-2032.
Protocol designing services encompass several key activities. This includes conducting a comprehensive literature review to identify the current standard of care, defining the primary and secondary endpoints, determining the sample size and statistical analysis plan, and ensuring compliance with relevant guidelines and regulations. The protocol designers also consider practical aspects such as patient recruitment, data collection, and monitoring requirements.
Site identification services are in high demand in the Europe clinical trials market. Pharmaceutical companies, contract research organizations (CROs), and sponsors often seek the expertise of experienced site identification specialists or specialized service providers. Several key activities are included in site identification services.
Europe Clinical Trials Market Country Insights
The Europe clinical trials market includes countries such as Germany, France, the UK, Italy, Spain, Austria, and the rest of Europe. The European clinical trials market is driven by several key factors such as the increasing number of clinical trials and strong healthcare infrastructure that contribute to its growth and development. Germany held the largest market share and France is expected to register the highest CAGR from 2023 to 2032.
The expansion of clinical trials is a significant driver of the European market. There has been a steady rise in the number of clinical trials conducted in Europe, driven by the growing demand for innovative therapies, advancements in medical research, and the need for evidence-based medicine. For instance, In the EU / EEA, approximately 4,000 clinical trials are carried out each year. Additionally, Europe has seen substantial investments in pharmaceutical Research & Development (R&D), which further drives the clinical trials market. Pharmaceutical companies and biotech firms are continually investing in developing new drugs, therapies, and medical devices. These investments not only support the conduct of clinical trials but also contribute to the growth of the healthcare industry. For instance, in 2022, the UK Government launched the Life Sciences Innovative Manufacturing Fund program aimed at supporting the manufacture of cutting-edge medical treatments, devices, and diagnostics.
Figure 3: EUROPE CLINICAL TRIALS MARKET BY COUNTRY 2023 & 2032 (USD Billion)
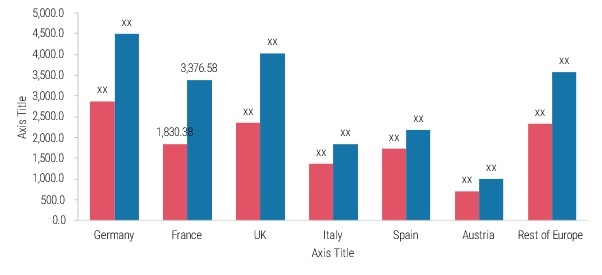
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Furthermore, Europe boasts a well-established research infrastructure, including top-tier academic institutions, renowned hospitals, and state-of-the-art Clinical Research Organizations (CROs). This infrastructure provides the necessary resources and expertise for conducting high-quality clinical trials. For instance, institutions like the University of Oxford (UK), Karolinska Institute (Sweden), and University College London (UK) are known for their contributions to clinical research and attract sponsors and researchers from around the world.
Europe Clinical Trials Market Key Market Players & Competitive Insights
The Europe clinical trials market is distinguished by the presence of numerous global, regional, and local players catering to the clinical trial techniques that are evolving at a rapid pace. Furthermore, the technological advancements in clinical trial services in Europe are further driving the growth of the Europe clinical trials market during the forecast period. The major players have adopted a strategy of obtaining regulatory approval from government agencies for their services and signing contracts and agreements to broaden their reach and reduce operational costs. The Europe clinical trials market is extremely competitive, with players competing, partnering, and investing heavily in research and development to gain a significant market share. The market is moderately fragmented with rising competition, increasing collaborative partnerships, and other strategic decisions to achieve operational efficiency.
ICON plc is a major player in Europe clinical trial market offering clinical development, consulting, and commercialization services including clinical research services. The clinical development services encompass all stages of development (Phases I-IV), both pre and post-approval, data solutions, as well as site and patient access services. Laboratory services offer a range of testing services, such as bioanalytical, biomarker, vaccine, good manufacturing practice (GMP), and central laboratory services.
Moreover in March 2023, ICON and LEO Pharma (Denmark) formed a strategic partnership to accelerate clinical trial execution in medical dermatology.
Key Companies Europe Clinical Trials market in market includes.
- Charles River Laboratories (US)
- Parexel International (MA) Corporation (US)
- ICON plc (Ireland)
- Laboratory Corporation of America Holdings (US)
- PPD Inc (US)
- Syneos Health (US)
- CTI Clinical Trial & Consulting (US)
- IQVIA (US)
- Medpace INC (US)
- Antaea Medical Services Ltd (Greece)
Europe Clinical Trials market Industry Developments
- February 2021, ICON plc (Ireland) has announced the availability of two new digital tools to assist clinical research sites and sponsors. TFIRECREST digital solutions improve measurable efficiency and quality in clinical trials and are used by 18 of the top 20 pharmaceutical companies, with over 540,000 users across 233 indications
- January 2020, Syneos Health (US) acquired Illingworth Research Group (England) to meet the growing demand for direct-to-patient services as well as the growing demand for in-home trials. As a result of the acquisition, Syneos Health's decentralized clinical trial solutions expand in scope and functionality
- May 2019, ICON plc (Ireland) announced that it has acquired a majority shareholding in MeDiNova Research (UK), a site network with research sites in key markets in Europe and Africa.
| Report Attribute/Metric |
Details |
| Market Size 2022 |
USD 13.18 Billion |
| Market Size 2023 |
USD 13.73 Billion |
| Market Size 2032 |
USD 23.47 Billion |
| Compound Annual Growth Rate (CAGR) |
7.94% (2024-2032) |
| Base Year |
2023 |
| Forecast Period |
2024-2032 |
| Historical Data |
2019 to 2021 |
| Forecast Units |
Value (USD Billion) |
| Report Coverage |
Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered |
Phase, Study Design, Service Type Application, and Country |
| Countries Covered |
Germany, France, UK, Italy, Spain, Austria, rest of Europe |
| Key Companies Profiled |
. Charles River Laboratories (US) Parexel International (MA) Corporation (US) ICON plc (Ireland) Laboratory Corporation of America Holdings (US) PPD Inc (US) Syneos Health (US) CTI Clinical Trial & Consulting (US) IQVIA (US) Medpace INC (US) Antaea Medical Services Ltd (Greece) |
| Key Market Opportunities |
· Digital Transformation in Clinical Trials |
| Key Market Drivers |
· Increasing Number of Clinical Trials · Strong Healthcare Infrastructure |
Frequently Asked Questions (FAQ) :
The Europe Clinical Trials market is anticipated to reach 24.7 billion during the forecast period of 2024-2032.
The Europe Clinical Trials market is expected to grow at an 7.94% CAGR during the forecast period from 2024 to 2032.
Germany is estimated to hold the largest market share in the Europe Clinical Trials market.
Charles River Laboratories (US), Parexel International (MA) Corporation (US), ICON plc (Ireland), Laboratory Corporation of America Holdings (US), PPD Inc (US), Syneos Health (US), CTI Clinical Trial & Consulting (US)
The Pharmaceutical segment led the Europe Clinical Trials market by application.